Controlled synthesis of NiSe2-NiMoO4-MoO3 material on nickel foam as an efficient hydrogen evolution reaction catalyst in seawater and urea electrolytes
IF 8.6
2区 工程技术
Q1 ENERGY & FUELS
引用次数: 0
Abstract
Hydrogen energy is considered as a new clean energy to replace traditional fossil energy. How to achieve large-scale industrial production has always been a problem that we are committed to studying. Hydrogen generation by electrolysis of water is considered to be one of the most effective hydrogen production approaches at present. However, with the global shortage of fresh water resources, we urgently need to prepare an efficient and low-cost seawater splitting catalyst. In this paper, the heterogeneous NiSe2-NiMoO4-MoO3 material was successfully synthesized on foamed nickel substrate through hydrothermal and calcination approaches. And it showed excellent hydrogen evolution reaction (HER) performance, in 1 M KOH + seawater solution, the current density of 10 mA cm−2 can be obtained with only overpotential of 105 mV. In 0.5 M urea+1 M KOH solution, a mere overpotential of 87 mV is required to drive a current density of 10 mA cm−2. In the stability test of 15 h, the activity of the catalyst material remained stable after a short decline, showing acceptable stability performance. Density functional theory (DFT) calculations proved that NiMoO4 plays a major role in the reaction and their synergistic catalysis results in better catalytic activity and stability. This study proposes a novel understanding for the preparation of HER catalyst with low cost and high efficiency.
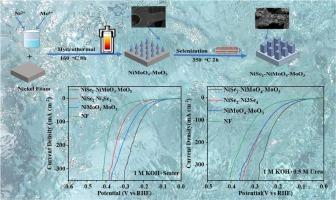
在泡沫镍上可控合成 NiSe2-NiMoO4-MoO3 材料,作为海水和尿素电解质中的高效氢进化反应催化剂
氢能被认为是替代传统化石能源的新型清洁能源。如何实现大规模工业化生产一直是我们致力于研究的问题。电解水制氢被认为是目前最有效的制氢方法之一。然而,随着全球淡水资源的短缺,我们迫切需要制备一种高效、低成本的海水裂解催化剂。本文通过水热法和煅烧法,在发泡镍基底上成功合成了异质 NiSe2-NiMoO4-MoO3 材料。在 1 M KOH + 海水溶液中,只需 105 mV 的过电位就能获得 10 mA cm-2 的电流密度。在 0.5 M 尿素 +1 M KOH 溶液中,只需要 87 mV 的过电位就能驱动 10 mA cm-2 的电流密度。在 15 小时的稳定性测试中,催化剂材料的活性在短暂下降后保持稳定,显示了可接受的稳定性能。密度泛函理论(DFT)计算证明,NiMoO4 在反应中发挥了重要作用,二者的协同催化作用可获得更好的催化活性和稳定性。这项研究为制备低成本、高效率的 HER 催化剂提出了一种新的认识。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Sustainable Materials and Technologies
Energy-Renewable Energy, Sustainability and the Environment
CiteScore
13.40
自引率
4.20%
发文量
158
审稿时长
45 days
期刊介绍:
Sustainable Materials and Technologies (SM&T), an international, cross-disciplinary, fully open access journal published by Elsevier, focuses on original full-length research articles and reviews. It covers applied or fundamental science of nano-, micro-, meso-, and macro-scale aspects of materials and technologies for sustainable development. SM&T gives special attention to contributions that bridge the knowledge gap between materials and system designs.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


