An intelligent poly aptamer-encoded DNA nanoclew for tumor site activated mitochondria-targeted photodynamic therapy and MR imaging
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Mitochondria-targeted photodynamic therapy (PDT) has emerged as one of the most promising antitumor therapies, as it significantly enhances the efficacy of photosensitizers. An efficient and biocompatible nanocarrier to deliver cationic photosensitizers (PSs) is vital for mitochondria-targeted PDT but still challenging. Herein, a poly-AS1411 aptamer DNA nanoclew (AS-AMD) synthesized via rolling circle amplification (RCA) is developed, incorporating mitochondria-targeted PSs (APNO) and paramagnetic Mn2+ for mitochondria-targeted PDT and magnetic resonance imaging (MRI). The AS1411 aptamer of AS-AMD has been engineered to enhance tumor targeting and cellular internalization. Paramagnetic Mn2+ released in the acidic tumor microenvironment promotes MRI performance of the tumor tissue and guides subsequent PDT. The released cationic APNO selectively targets the mitochondrial membrane and generates reactive oxygen species (ROS) that induce the apoptosis of 4T1 breast tumor cells. Additionally, AS-AMD exhibits effective tumor targeting in the 4T1-tumor-bearing mice model, significantly enhanced MRI performance and PDT efficacy. Therefore, this study introduces an interesting strategy to achieve efficient mitochondrial-targeted delivery of cationic PSs and provides a versatile biocompatible DNA nanoplatform for the development of nanotheranostic agents.
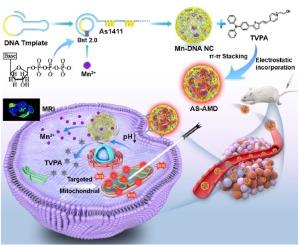
用于肿瘤部位激活线粒体靶向光动力疗法和核磁共振成像的智能多肽合剂编码 DNA 纳米螯合剂
线粒体靶向光动力疗法(PDT)已成为最有前途的抗肿瘤疗法之一,因为它能显著提高光敏剂的疗效。一种高效、生物相容性好的纳米载体可递送阳离子光敏剂(PSs),这对线粒体靶向光动力疗法至关重要,但仍具有挑战性。本文开发了一种通过滚圆扩增(RCA)合成的聚-AS1411适配体DNA纳米载体(AS-AMD),它结合了线粒体靶向光敏剂(APNO)和顺磁性Mn2+,用于线粒体靶向PDT和磁共振成像(MRI)。AS-AMD的AS1411适配体经过设计,可增强肿瘤靶向性和细胞内化。在酸性肿瘤微环境中释放的顺磁性 Mn2+ 可提高肿瘤组织的磁共振成像性能,并指导后续的局部放疗。释放的阳离子 APNO 可选择性地靶向线粒体膜,产生活性氧 (ROS),诱导 4T1 乳腺肿瘤细胞凋亡。此外,AS-AMD 在 4T1 肿瘤小鼠模型中表现出有效的肿瘤靶向性,显著提高了磁共振成像性能和光动力疗法的疗效。因此,本研究提出了一种有趣的策略来实现阳离子 PSs 的高效线粒体靶向递送,并为纳米otheranostic 药剂的开发提供了一种多功能的生物相容性 DNA 纳米平台。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


