Impact of the key residues of 1-Deoxy-D-Xylulose-5-Phosphate synthase on its catalysis
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
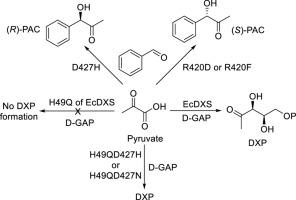
1-Deoxy-D-xylulose-5-phosphate synthase (DXS) is the first rate-limiting enzyme in the 2-methyl-D-erythritol-4-phosphate (MEP) terpenoid biosynthetic pathway. To explore the active sites of the protein, we made a series of mutations to the key amino acid residues of Escherichia coli DXS including H49, R420, D427, and R478. The results show that several mutants including H49Q lose almost all their catalytic activity in DXP synthesis, but the double-point mutants H49QD427H and H49QD427 N recover 100 % activity of the wild type enzyme. The kinetic characterization displays that the affinity of the double-point mutants for pyruvate and D-glyceraldehyde-3-phosphate only slightly decreases. We also assessed impact of the mutation on the oxidative decarboxylation activity of all the mutants. Despite the fact that H49QD427H and H49QD427 N get back the full activity with regard to DXP synthesis, their oxidase activity remains unrestored. Furthermore, we assayed the acceptor substrate spectrum of the mutants and the results show that a large portion of them catalyzes ligation between pyruvate and nitrosobenzene to form N-phenyl-N-hydroxyacetamide, whereas R420D, R420F, and D427H exhibit activity in connection of pyruvate and benzaldehyde to afford phenylacetocarbinol (PAC), with the first two mutants yielding (S)-PAC (yields 21 %-25 %, ee values 71–75 %) while D427H furnishing (R)-PAC (yield 41 %, ee value 91 %), implying the key role of D427 for the acceptor substrate recognition and the stereoselectivity of the enzyme. It is worth noting that this is the first report of DXS mutants that can recognize benzaldehyde as an acceptor substrate and generate both enantiomers of PAC.
1-Deoxy-D-Xylulose-5-Phosphate synthase 的关键残基对其催化作用的影响
1-脱氧-D-木酮糖-5-磷酸合成酶(DXS)是 2-甲基-D-赤藓糖醇-4-磷酸(MEP)萜类化合物生物合成途径中的第一个限速酶。为了探索该蛋白的活性位点,我们对大肠杆菌 DXS 的 H49、R420、D427 和 R478 等关键氨基酸残基进行了一系列突变。结果表明,包括 H49Q 在内的几个突变体几乎完全丧失了合成 DXP 的催化活性,但双点突变体 H49QD427H 和 H49QD427 N 的活性恢复到了野生型酶的 100%。动力学特征显示,双点突变体对丙酮酸和 D-甘油醛-3-磷酸的亲和力仅略有下降。我们还评估了突变对所有突变体氧化脱羧活性的影响。尽管 H49QD427H 和 H49QD427 N 恢复了合成 DXP 的全部活性,但它们的氧化酶活性仍然没有恢复。此外,我们还测定了突变体的受体底物谱,结果表明,它们中的大部分催化丙酮酸与亚硝基苯之间的连接,形成 N-苯基-N-羟基乙酰胺,而 R420D、R420F 和 D427H 则在丙酮酸与苯甲醛的连接中表现出活性,生成苯乙酰卡宾醇(PAC)、前两个突变体产生 (S)-PAC(产率 21%-25%,ee 值 71-75%),而 D427H 产生 (R)-PAC(产率 41%,ee 值 91%),这意味着 D427 在接受底物识别和酶的立体选择性方面起着关键作用。值得注意的是,这是首次报道 DXS 突变体能够识别苯甲醛作为受体底物并生成 PAC 的两种对映体。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


