Use of red-mud (MRM) in industrial catalysis: Selective conversion of biomass derived furfural to furfuryl alcohol using Ni-MRM – preparation, characterization and activity studies – elucidating mechanism of hydrogenation using DFT
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
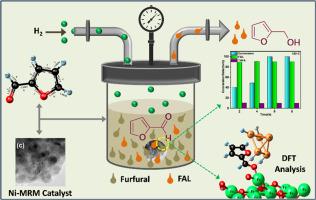
Conversion of furfural (FFL) to furfuryl alcohol (FOL) using nickel loaded modified red mud (Ni-MRM) was developed with an aim to eliminate toxic Cu-Cr catalyst or precious metal catalyst currently used industrially. Ni-MRM was characterized using various advanced instruments such as XRD, TEM, TPR, BET and XPS. Thermal stability of the material was computed using Kissinger-Akahira-Sonuse (KAS) model. Ni-MRM catalytic activity was studied with variation of parameters. A 15 % Ni-loading on MRM (Ni(15 %)-MRM) shows significant product selectivity (>90 %) in optimized reaction conditions. Density functional theory (DFT) was used for calculation of adsorption energy, charge of the metal at the material active sites. The theoretical calculation shows that H2 molecule adsorbed upon electron rich Ni surface facilitate bond cleavage to initiate the reaction with adsorbed furfural molecule. Ni-MRM could be reused without significant loss of material catalytic activity for production of furfuryl alcohol. The work also shows effective utilization of properties of red mud for selective transformation of furfural, a bio-renewable material, to value added products.
在工业催化中使用红泥(MRM):利用 Ni-MRM 将生物质衍生的糠醛选择性转化为糠醇--制备、表征和活性研究--利用 DFT 阐明氢化机理
开发了使用镍负载改性赤泥(Ni-MRM)将糠醛(FFL)转化为糠醇(FOL)的方法,旨在消除目前工业上使用的有毒铜-铬催化剂或贵金属催化剂。使用 XRD、TEM、TPR、BET 和 XPS 等各种先进仪器对 Ni-MRM 进行了表征。利用基辛格-阿卡希拉-索努塞(KAS)模型计算了材料的热稳定性。随着参数的变化,对 Ni-MRM 催化活性进行了研究。在优化的反应条件下,MRM(Ni(15%)-MRM)上 15% 的镍负载显示出显著的产物选择性(90%)。密度泛函理论(DFT)用于计算材料活性位点的吸附能和金属电荷。理论计算表明,吸附在富电子镍表面的 H2 分子会促进键的裂解,从而引发与吸附的糠醛分子的反应。Ni-MRM 可以重复使用,不会明显降低材料在生产糠醇时的催化活性。这项研究还表明,可以有效利用赤泥的特性,将糠醛这种生物可再生材料有选择地转化为高附加值产品。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


