Heterogeneous visible-light photoredox catalysis with NiCl2@g-C3N4 for induced C(sp2)-H/NH cross-dehydrogenative coupling
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
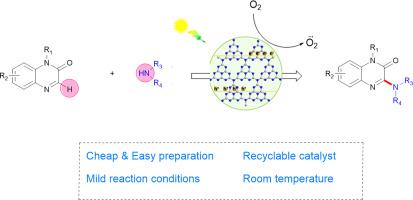
A novel photoactive NiCl2@g-C3N4 catalyst system, which effectively drives C(sp2)-H/N![]() H bond formation between quinolin-2(1H)-ones, primary and secondary aliphatic amines under blue light irradiation. The NiCl2@g-C3N4 composite with a NiCl2 loading of 0.7wt% had the highest photoactivity, and the calculated band gap energy value is 2.56 eV The nickel ion could act as an electron acceptor enhancing carrier separation and transfer efficiency, greatly enhanced the photoreaction efficiency of g-C3N4. Preliminary mechanistic studies indicate upon visible light irradiation, the photo-generated electrons and holes act as oxidants, thereby generating amine radicals in the absence of external chemical oxidants. This provides a straightforward and efficient approach for directly 3-aminating quinoxalines-2(1H)-ones. Moreover, the heterogeneous catalyst can be recycled at least six times without significant activity loss.
H bond formation between quinolin-2(1H)-ones, primary and secondary aliphatic amines under blue light irradiation. The NiCl2@g-C3N4 composite with a NiCl2 loading of 0.7wt% had the highest photoactivity, and the calculated band gap energy value is 2.56 eV The nickel ion could act as an electron acceptor enhancing carrier separation and transfer efficiency, greatly enhanced the photoreaction efficiency of g-C3N4. Preliminary mechanistic studies indicate upon visible light irradiation, the photo-generated electrons and holes act as oxidants, thereby generating amine radicals in the absence of external chemical oxidants. This provides a straightforward and efficient approach for directly 3-aminating quinoxalines-2(1H)-ones. Moreover, the heterogeneous catalyst can be recycled at least six times without significant activity loss.
利用NiCl2@g-C3N4进行异相可见光光氧化催化,诱导C(sp2)-H/NH交叉脱氢偶联
一种新型光活性 NiCl2@g-C3N4 催化剂体系,可在蓝光照射下有效驱动喹啉-2(1H)-酮、伯胺和仲胺之间形成 C(sp2)-H/NH 键。镍含量为 0.7wt% 的 NiCl2@g-C3N4 复合材料具有最高的光活性,其计算带隙能值为 2.56 eV。初步的机理研究表明,在可见光照射下,光产生的电子和空穴可作为氧化剂,从而在没有外部化学氧化剂的情况下产生胺自由基。这为直接 3-氨基化喹喔啉-2(1H)-酮提供了一种直接而有效的方法。此外,这种异相催化剂可以循环使用至少六次,而不会有明显的活性损失。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


