Development of an efficient biocatalytic three-component reaction for synthesizing pyrrole derivatives
IF 3.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Multi-component reactions (MCRs) enable the development of efficient and atom-economic methods for synthesizing pyrrole derivatives. However, the current MCRs methods for synthesizing pyrrole derivatives in the enzymatic process have been unexploited. Herein, we developed a one-pot three-component enzymatic promiscuous catalytic system with green, efficient, and atom-economic to construct pyrrole derivatives. In this system, amines and 1,3-dicarbonyl compounds formed enamines with subsequent Michael addition with nitroolefins catalyzed by trypsin. A series of pyrrole derivatives were successfully obtained with moderate to good yield (up to 92 %). In addition, molecular simulations were conducted to gain insight into the source of differing tolerance of aromatic amides and fatty amines and the intrinsic effect of solvents in this system. This enzymatic catalytic one-pot three-component system has excellent functional tolerance, simple operation, and application potential for industrial production demonstrated by the gram scale experiment.
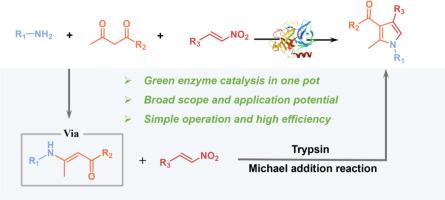
开发合成吡咯衍生物的高效生物催化三组分反应
多组分反应(MCRs)有助于开发高效、原子经济的吡咯衍生物合成方法。然而,目前在酶法工艺中合成吡咯衍生物的多组分反应方法尚未得到开发。在此,我们开发了一种绿色、高效、原子经济的一锅三组分酶法杂化催化体系来构建吡咯衍生物。在该系统中,胺和 1,3-二羰基化合物形成烯胺,随后在胰蛋白酶的催化下与硝基烯烃发生迈克尔加成反应。成功获得了一系列吡咯衍生物,收率从中等到良好(高达 92%)。此外,还进行了分子模拟,以深入了解芳香族酰胺和脂肪胺耐受性不同的原因以及溶剂在该体系中的内在影响。该酶催化单锅三组分系统具有极佳的功能耐受性,操作简单,克级实验证明了其在工业生产中的应用潜力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


