Promoting removal of polystyrene microplastics from wastewater by electrochemical treatment
IF 6.3
2区 工程技术
Q1 ENGINEERING, CHEMICAL
引用次数: 0
Abstract
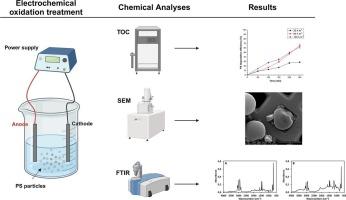
Microplastics (MPs) are emerging contaminants with potential ecological and human health impacts, necessitating effective remediation technologies. Recently, electrochemical oxidation (EO) has garnered attention as a suitable method for treating water contaminated with MPs. However, research on EO's effectiveness remains limited. This study investigates the EO treatment of 1.0 μm polystyrene (PS) MPs in a lab-scale reactor using boron-doped diamond (BDD) electrodes. Various operational parameters, such as electrolyte composition and concentration, initial PS concentration, and applied current density, were examined for their impact on PS degradation efficiency. Optimal degradation was achieved using Na2SO4 (0.02 M) as a supporting electrolyte, an initial PS concentration of 60 mg L−1, and an applied current density of 60 A/m2 for 5 h. The degradation mechanism likely involved indirect EO through the formation of highly oxidizing radicals rather than direct EO between the anode and PS molecules. High current densities induced morphological changes in the PS microparticles. Fourier transform infrared spectroscopy confirmed new functional groups on the PS surface, indicating oxidation. These findings suggest that EO using BDD electrodes is a promising approach for treating microplastic-polluted water. However, further studies are needed to optimize the process, particularly concerning power requirements, electrode costs, and reactor configuration.
通过电化学处理促进去除废水中的聚苯乙烯微塑料
微塑料(MPs)是一种新出现的污染物,对生态和人类健康具有潜在影响,因此需要有效的修复技术。最近,电化学氧化(EO)作为一种处理受 MPs 污染的水的合适方法引起了人们的关注。然而,有关 EO 效果的研究仍然有限。本研究采用掺硼金刚石(BDD)电极,在实验室规模的反应器中对 1.0 μm 聚苯乙烯(PS)MPs 进行电化学氧化处理。研究了各种操作参数对 PS 降解效率的影响,如电解质成分和浓度、PS 初始浓度和应用电流密度。采用 Na2SO4(0.02 M)作为支撑电解质、PS 初始浓度为 60 mg L-1、施加电流密度为 60 A/m2 持续 5 小时,实现了最佳降解效果。降解机理可能是通过形成高度氧化自由基进行间接氧化还原,而不是阳极与 PS 分子之间直接氧化还原。高电流密度导致 PS 微颗粒发生形态变化。傅立叶变换红外光谱证实了 PS 表面的新官能团,表明其已被氧化。这些发现表明,使用 BDD 电极进行环氧乙烷氧化是一种很有前景的处理微塑料污染水的方法。不过,还需要进一步研究来优化该工艺,特别是在功率要求、电极成本和反应器配置方面。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of water process engineering
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
10.70
自引率
8.60%
发文量
846
审稿时长
24 days
期刊介绍:
The Journal of Water Process Engineering aims to publish refereed, high-quality research papers with significant novelty and impact in all areas of the engineering of water and wastewater processing . Papers on advanced and novel treatment processes and technologies are particularly welcome. The Journal considers papers in areas such as nanotechnology and biotechnology applications in water, novel oxidation and separation processes, membrane processes (except those for desalination) , catalytic processes for the removal of water contaminants, sustainable processes, water reuse and recycling, water use and wastewater minimization, integrated/hybrid technology, process modeling of water treatment and novel treatment processes. Submissions on the subject of adsorbents, including standard measurements of adsorption kinetics and equilibrium will only be considered if there is a genuine case for novelty and contribution, for example highly novel, sustainable adsorbents and their use: papers on activated carbon-type materials derived from natural matter, or surfactant-modified clays and related minerals, would not fulfil this criterion. The Journal particularly welcomes contributions involving environmentally, economically and socially sustainable technology for water treatment, including those which are energy-efficient, with minimal or no chemical consumption, and capable of water recycling and reuse that minimizes the direct disposal of wastewater to the aquatic environment. Papers that describe novel ideas for solving issues related to water quality and availability are also welcome, as are those that show the transfer of techniques from other disciplines. The Journal will consider papers dealing with processes for various water matrices including drinking water (except desalination), domestic, urban and industrial wastewaters, in addition to their residues. It is expected that the journal will be of particular relevance to chemical and process engineers working in the field. The Journal welcomes Full Text papers, Short Communications, State-of-the-Art Reviews and Letters to Editors and Case Studies
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


