Priming of cancer-immunity cycle by alleviating hypoxia-induced ferroptosis resistance and immunosuppression
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
Abstract
Stimulating a robust cancer-immunity cycle (CIC) holds promising potential for eliciting potent and enduring immune responses for cancer immunotherapy. However, designing a therapeutic nanomaterial capable of both enhancing tumor immunogenicity and mitigating immunosuppression is challenging and often associated with complicated design paradigms and immune-related adverse effects. Herein, a multienzyme-mimetic alloy nanosheet incorporating palladium (Pd) and iron (Fe) is developed, which can prime effective CIC by overcoming ferroptosis resistance for enhancing tumor immunogenicity and reprograming the tumor microenvironment for enhanced second near-infrared (NIR-II) photoimmunotherapy. The nanosheets accumulate in tumors when administered intravenously and counteract hypoxia through catalase-like oxygen production and subsequent reduction of hypoxia-inducible factor-1α, M2-like macrophages, regulatory T-cell, and programmed death-ligand 1 (PD-L1) expression. The surface plasmon resonance of the nanosheets enables NIR-II phototherapy and photoacoustic imaging, coupling with its ferroptosis and tumor microenvironment reprogram properties to synergize with anti-PD-L1 checkpoint blockade therapy to achieve satisfactory antitumor outcome. This study offers a strategy for localized tumor treatment and boosting the CIC through a straightforward and inexpensive nanomaterial design.
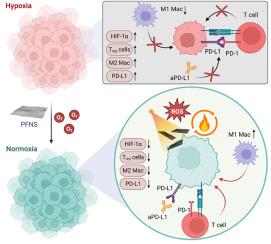
通过缓解缺氧诱导的铁蛋白沉积抵抗和免疫抑制,启动癌症-免疫循环
激发强大的癌症免疫循环(CIC)有望为癌症免疫疗法带来强效持久的免疫反应。然而,设计一种既能增强肿瘤免疫原性又能减轻免疫抑制的治疗性纳米材料具有挑战性,而且往往涉及复杂的设计范式和与免疫相关的不良反应。在此,我们开发了一种包含钯(Pd)和铁(Fe)的多酶模拟合金纳米片,它可以通过克服铁变态反应抗性来增强肿瘤免疫原性,并重新编程肿瘤微环境以增强第二次近红外(NIR-II)光免疫疗法,从而为有效的CIC提供能量。静脉注射纳米片时,纳米片会在肿瘤内积聚,并通过催化酶样产氧作用抵消缺氧,进而减少缺氧诱导因子-1α、M2 样巨噬细胞、调节性 T 细胞和程序性死亡配体 1 (PD-L1) 的表达。纳米片的表面等离子体共振可实现近红外-II光疗和光声成像,再加上其铁蛋白沉降和肿瘤微环境重编程特性,可与抗PD-L1检查点阻断疗法协同作用,达到令人满意的抗肿瘤效果。这项研究提供了一种通过简单、廉价的纳米材料设计进行肿瘤局部治疗和增强 CIC 的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


