In vivo biocompatibility assessment of 3D printed bioresorbable polymers for brain tissue regeneration. A feasibility study
IF 3.4
3区 环境科学与生态学
Q3 CELL & TISSUE ENGINEERING
引用次数: 0
Abstract
Introduction
The limited capacity of brain tissue to regenerate after acute injury, hampered by cell death, edema and inflammation, has led to an interest in promising and innovative approaches such as implantable regenerative scaffolds designed to improve brain plasticity. Leveraging the capabilities of bioprinting, these scaffolds can be tailored to match the intricate architecture of the brain.
Methods
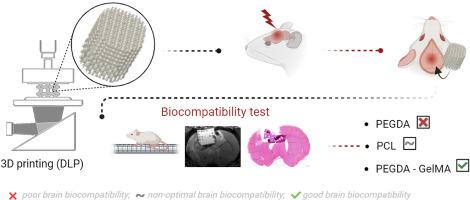
In this methodological study, we performed in vivo biocompatibility assessments after a brain lesion on three distinct bioeliminable or bioresorbable materials: Poly(ethylene glycol) diacrylate (PEGDA), Polycaprolactone (PCL) and a PEGDA mixed with gelatin methacrylate (PEGDA-GelMA).
Results
A scaffold with a complex shape was printed with patterns, spatial resolution and porosity adapted to cerebral cortex reconstruction. In vivo evaluations were complemented by behavioral monitoring, affirming the safety of these materials. High-resolution T2 MRI imaging effectively captured scaffold structures and demonstrated their non-invasive utility in monitoring degradability. ASL MRI imaging quantified cerebral blood flow and was positively and significantly correlated with lectin immunofluorescent labeling. It may be used to non-invasively monitor progressive revascularization of implants.
PEGDA produced an intense foreign-body response, encapsulated by a fibro-inflammatory barrier. On the other hand, PCL provoked a controlled inflammatory reaction and facilitated cell migration into the scaffold, although it induced a fibrotic response around PCL fibers. Conversely, the PEGDA-GelMA composite emerged as a promising candidate for intracerebral implantation. It facilitated the creation of a permissive glial layer, while also inducing neovascularization and attracting neuronal progenitors.
Conclusion
Behavior, MRI monitoring and histology allowed a thorough following of biomaterial biocompatibility. The collective findings position PEGDA-GelMA as a convincing biomaterial option as a basis for treating severe brain lesions, offering new avenues in the search for effective treatments.
用于脑组织再生的 3D 打印生物可吸收聚合物的体内生物相容性评估。可行性研究
引言由于细胞死亡、水肿和炎症等因素的影响,脑组织在急性损伤后的再生能力有限,因此人们开始关注前景广阔的创新方法,如旨在改善大脑可塑性的可植入再生支架。在这项方法学研究中,我们在脑损伤后对三种不同的生物可消化或生物可吸收材料进行了体内生物相容性评估:结果印制出了具有复杂形状的支架,其图案、空间分辨率和孔隙率均适合大脑皮层的重建。体内评估与行为监测相辅相成,证实了这些材料的安全性。高分辨率 T2 核磁共振成像有效捕捉了支架结构,并证明了其在监测降解性方面的无创实用性。ASL MRI 成像可量化脑血流,并与凝集素免疫荧光标记呈显著正相关。ASL MRI 成像可量化脑血流,并与凝集素免疫荧光标记呈显著正相关,可用于无创监测植入物的逐步血管再通。另一方面,PCL 会引发受控的炎症反应,并促进细胞迁移到支架中,但会在 PCL 纤维周围引起纤维化反应。相反,PEGDA-GelMA 复合材料成为脑内植入的理想候选材料。结论通过行为学、核磁共振成像监测和组织学研究,可以全面了解生物材料的生物相容性。这些研究结果使 PEGDA-GelMA 成为治疗严重脑损伤的一种令人信服的生物材料,为寻找有效的治疗方法提供了新的途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Regenerative Therapy
Engineering-Biomedical Engineering
CiteScore
6.00
自引率
2.30%
发文量
106
审稿时长
49 days
期刊介绍:
Regenerative Therapy is the official peer-reviewed online journal of the Japanese Society for Regenerative Medicine.
Regenerative Therapy is a multidisciplinary journal that publishes original articles and reviews of basic research, clinical translation, industrial development, and regulatory issues focusing on stem cell biology, tissue engineering, and regenerative medicine.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


