Morphology studies, optic proprieties, hirschfeld electrostatic potential mapping, docking molecular anti-inflammatory, and dynamic molecular approaches of hybrid phosphate
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
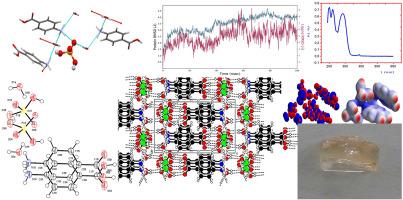
The above current study intends to identify new prospects for developing viable epilepsy treatments. To attain this purpose, the created P-carboxylammonium di-hydrogen monohydrate called (I). The research reveals the existence of both intermolecular (O–H⋯O) as well as N–H⋯O intramolecular hydrogen bonding in crystal packing patterns. As the fingerprint plots illustrate the different sorts of interactions and the hybrid system's relative abundance of each, However, the molecular docking results clearly demonstrate five typical hydrogen bonds, with the best binding posture of −4.757 kcal/mol for Lys244, Val272, Arg241, and Glu273 proteins when docked with (I) ligand. As a result, we may deduce that if the (I) ligand is a pharmaceutical used to treat epilepsy, it will probably be more potent than the conventional medication. As a result, (I) was simulated using molecular dynamics (MD) and is proposed as a viable therapeutic target for antiepileptic therapy. Reduced Density Gradient (RDG) analysis, highlighted the presence of significant non-covalent interactions (NCI) that contribute to the stability and structural integrity of the compound, emphasizing the importance of these interactions in the context of its potential applications, particularly in drug design and molecular interactions. Finally, the ELF and LOL analyses collectively enhance the understanding of the electronic structure of compound I, revealing critical information about electron distribution, localization, and the nature of interactions within the molecular framework. These insights are essential for predicting the compound's reactivity and potential applications in fields such as pharmaceuticals and materials science.
混合磷酸盐的形态学研究、光学特性、赫希菲尔德静电位图、对接分子消炎和动态分子方法
上述研究旨在确定开发可行的癫痫治疗方法的新前景。为实现这一目的,研究人员创造了名为(I)的对羧基铵二氢一水合物。研究揭示了晶体堆积模式中分子间(O-H⋯O)和分子内(N-H⋯O)氢键的存在。指纹图谱显示了不同种类的相互作用以及杂交体系中每种相互作用的相对丰度。然而,分子对接结果清楚地显示了五种典型的氢键,Lys244、Val272、Arg241 和 Glu273 蛋白质与(I)配体对接时的最佳结合姿态为-4.757 kcal/mol。因此,我们可以推断,如果(I)配体是一种用于治疗癫痫的药物,那么它的药效可能会比传统药物更强。因此,我们利用分子动力学(MD)模拟了 (I) 配体,并提出将其作为抗癫痫疗法的可行治疗靶点。还原密度梯度(RDG)分析凸显了显著的非共价相互作用(NCI)的存在,这有助于提高化合物的稳定性和结构完整性,强调了这些相互作用在其潜在应用中的重要性,特别是在药物设计和分子相互作用方面。最后,ELF 和 LOL 分析共同增进了对化合物 I 电子结构的了解,揭示了分子框架内电子分布、定位和相互作用性质的关键信息。这些见解对于预测化合物的反应性以及在制药和材料科学等领域的潜在应用至关重要。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


