Rituximab induces ferroptosis and RSL3 overcomes rituximab resistance in diffuse large B-cell lymphoma cells
IF 3.8
3区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Diffuse large B-cell lymphoma (DLBCL) is the most common malignant lymphoma in adults, and the use of rituximab has greatly improved the survival of DLBCL patients. Currently, the first-line treatment regimen for DLBCL is still rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone (R-CHOP), which significantly improves outcomes for DLBCL patients. However, a percentage of patients still experience refractory or relapsed disease. Since Dr. Brent R Stockwell proposed ferroptosis in 2012, Roudkenar, M. H. Roushandeh, A. M. Valashedi, M. R. and others proved the importance of ferroptosis in cancer drug resistance. The purpose of this study was to elucidate whether rituximab could exert anticancer effects on DLBCL cells by promoting ferroptosis. Cell viability was assessed using the Cell Counting Kit-8. The results showed that rituximab exposure induced ferroptosis in OCI-LY1 cells. However, combination with ferroptosis inhibitor ferrostatin (Fer-1) rescued ferroptosis-induced injury, indicating that ferroptosis plays a key role in rituximab-induced cell death. Western blotting was performed to detect the levels of specific ferroptosis-associated proteins in DLBCL. Moreover, GSH depletion and MDA upregulation was assessed using GSH assays and MDA assay kits in rituximab-treated OCI-LY1 cells. In addition, rituximab failed to induce ferroptosis in rituximab-resistant cell lines. Treatment with RSL3 enhanced the effects of rituximab on DLBCL cells by inhibiting cell viability. In conclusion, we report for the first time that rituximab induces ferroptosis in lymphoma cells, at least partially through the SLC7A11/GPX4 axis. We also identify targeting ferroptosis as a promising therapeutic option for both sensitive cells and resistant cells in the treatment of DLBCL.
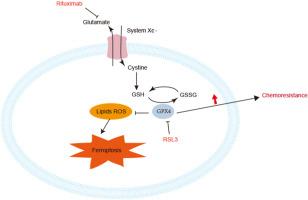
利妥昔单抗可诱导弥漫大 B 细胞淋巴瘤细胞发生铁变态反应,RSL3 可克服利妥昔单抗的抗药性
弥漫大B细胞淋巴瘤(DLBCL)是成人中最常见的恶性淋巴瘤,利妥昔单抗的使用大大提高了DLBCL患者的生存率。目前,DLBCL 的一线治疗方案仍然是利妥昔单抗加环磷酰胺、多柔比星、长春新碱和泼尼松龙(R-CHOP),这能显著改善 DLBCL 患者的预后。然而,仍有一部分患者会出现难治性或复发性疾病。自布伦特-R-斯托克韦尔(Brent R Stockwell)博士于2012年提出铁凋亡以来,Roudkenar、M. H. Roushandeh、A. M. Valashedi、M. R. 等人证明了铁凋亡在癌症耐药性中的重要性。本研究的目的是阐明利妥昔单抗是否能通过促进铁氧化作用对 DLBCL 细胞产生抗癌作用。细胞活力采用细胞计数试剂盒-8进行评估。结果表明,利妥昔单抗暴露可诱导 OCI-LY1 细胞发生铁变态反应。然而,与铁蛋白沉积抑制剂铁前列素(Fer-1)联合使用可挽救铁蛋白沉积诱导的损伤,这表明铁蛋白沉积在利妥昔单抗诱导的细胞死亡中起着关键作用。研究人员用 Western 印迹法检测了 DLBCL 中特定的铁凋亡相关蛋白的水平。此外,在利妥昔单抗处理的OCI-LY1细胞中,使用GSH检测试剂盒和MDA检测试剂盒对GSH消耗和MDA上调进行了评估。此外,在利妥昔单抗耐药细胞系中,利妥昔单抗未能诱导铁变态反应。用 RSL3 处理可抑制细胞活力,从而增强利妥昔单抗对 DLBCL 细胞的作用。总之,我们首次报道了利妥昔单抗在淋巴瘤细胞中诱导铁突变,至少部分是通过 SLC7A11/GPX4 轴。我们还发现,在治疗 DLBCL 的过程中,针对敏感细胞和耐药细胞的铁突变是一种很有前景的治疗选择。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Archives of biochemistry and biophysics
生物-生化与分子生物学
CiteScore
7.40
自引率
0.00%
发文量
245
审稿时长
26 days
期刊介绍:
Archives of Biochemistry and Biophysics publishes quality original articles and reviews in the developing areas of biochemistry and biophysics.
Research Areas Include:
• Enzyme and protein structure, function, regulation. Folding, turnover, and post-translational processing
• Biological oxidations, free radical reactions, redox signaling, oxygenases, P450 reactions
• Signal transduction, receptors, membrane transport, intracellular signals. Cellular and integrated metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


