Recent electrochemical applications of Two-Dimensional nanoclays based materials
IF 4.9
2区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
Abstract
Naturally-occurring mineral substances called clays are available at a low cost and are ecologically beneficial. These clays are composed of two-dimensional (2D) nanoclays, layered silicates made up of platelets with nanoscale thickness joined together by van der Waals forces. There are various types of nanoclays frequently cited in the literature, such as montmorillonite (MMT), kaolinite, vermiculite (VMT), laponite, and illite. These nanoclays possess distinct physical and chemical characteristics, which make them useful for a variety of applications in scientific and industrial fields. This study includes the development of electrochemical sensors that employ modified electrodes with 2D nanoclays and their composites to detect drugs, diagnose medical conditions, monitor environmental pollutants, and ensure food safety. This article discusses these applications in detail. Furthermore, we explored the uses of 2D nanoclays in various electrochemical applications, such as serving as cathodes in Li-sulfur batteries, separators in batteries and supercapacitors, and acting the role of catalysts in the processes of water electrolysis and oxygen reduction reaction. Finally, we assessed the current challenges related to the use of 2D nanoclays in electrochemistry.
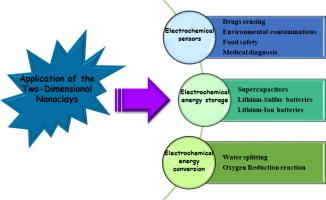
基于二维纳米粘土材料的最新电化学应用
被称为粘土的天然矿物质成本低廉,而且对生态有益。这些粘土由二维(2D)纳米粘土组成,是由具有纳米级厚度的小板通过范德华力连接在一起的层状硅酸盐。文献中经常提到的纳米粘土有多种类型,如蒙脱土 (MMT)、高岭石、蛭石 (VMT)、青石和伊利石。这些纳米粘土具有独特的物理和化学特性,可用于科学和工业领域的各种应用。这项研究包括开发采用二维纳米粘土及其复合材料改性电极的电化学传感器,以检测药物、诊断病情、监测环境污染物和确保食品安全。本文将详细讨论这些应用。此外,我们还探讨了二维纳米黏土在各种电化学应用中的用途,如用作锂硫电池的阴极、电池和超级电容器的隔膜,以及在水电解和氧还原反应过程中发挥催化剂的作用。最后,我们评估了当前在电化学中使用二维纳米粘土所面临的挑战。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microchemical Journal
化学-分析化学
CiteScore
8.70
自引率
8.30%
发文量
1131
审稿时长
1.9 months
期刊介绍:
The Microchemical Journal is a peer reviewed journal devoted to all aspects and phases of analytical chemistry and chemical analysis. The Microchemical Journal publishes articles which are at the forefront of modern analytical chemistry and cover innovations in the techniques to the finest possible limits. This includes fundamental aspects, instrumentation, new developments, innovative and novel methods and applications including environmental and clinical field.
Traditional classical analytical methods such as spectrophotometry and titrimetry as well as established instrumentation methods such as flame and graphite furnace atomic absorption spectrometry, gas chromatography, and modified glassy or carbon electrode electrochemical methods will be considered, provided they show significant improvements and novelty compared to the established methods.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


