Control over electronic structures of organic diradicaloids via precise B/O-heterocycle fusion
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
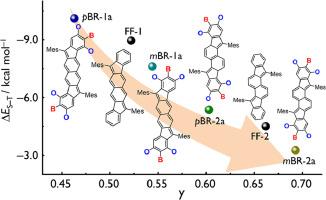
Diradicaloid polycyclic hydrocarbons (PHs) own unique open-shell electronic structures and exhibit potential utility in the fields of organic electronics and spintronics. Herein, we disclose precise fusion of B/O-heterocycles onto PHs for control over their electronic structures and diradical properties. We designed and synthesized four B/O-containing diradicaloid isomers that feature the fluoreno[3,2-b]fluorene and fluoreno[2,1-a]fluorene π-skeletons, respectively. The precise B/O-heterocycle fusion modes along with the changed conjugation patterns lead to their modulated electronic structures and properties, such as diradical and aromatic structures, energy levels and band gaps, as well as magnetic, electrochemical and photophysical properties. Notably, the mode A may decrease the open-shell extent, whereas the mode B can enhance the diradical nature, leading to their well-tuned diradical characters in the range of 0.46‒0.70. Moreover, the mode A stabilizes the LUMOs and the mode B obviously increases the HOMO levels, which are remarkably contributed by the B and O atoms, respectively, further giving rise to the decreased band gaps and redshifted absorptions. This study clearly illustrates the electronic effects of B/O-heterocycle fusion on PHs and gains insight into B/O-type organic diradicaloids. These findings will provide an important guideline for the design of more fascinating heteroatom-containing diradicaloids.
通过精确的 B/O 异环融合控制有机二环化合物的电子结构
二拉环多环碳氢化合物(PHs)具有独特的开壳电子结构,在有机电子学和自旋电子学领域具有潜在用途。在此,我们揭示了如何将 B/O 异环化合物精确地融合到 PHs 上,以控制其电子结构和二维性质。我们设计并合成了四种含 B/O 的类二维异构体,它们分别具有芴并[3,2-b]芴和芴并[2,1-a]芴 π-骨架。精确的 B/O 杂环融合模式以及共轭模式的改变导致了它们的电子结构和特性发生了变化,如二元和芳香结构、能级和带隙,以及磁性、电化学和光物理特性。值得注意的是,模式 A 可以降低开壳程度,而模式 B 则可以增强二元性质,从而使它们的二元特性在 0.46-0.70 范围内得到很好的调节。此外,模式 A 稳定了 LUMO,而模式 B 则明显提高了 HOMO 水平,这分别是由 B 原子和 O 原子显著贡献的,从而进一步导致了带隙的减小和吸收的红移。这项研究清楚地说明了 B/O 异环融合对 PH 的电子效应,并深入了解了 B/O 型有机二维化合物。这些发现将为设计更多迷人的含杂原子的二维类化合物提供重要指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


