Bifunctional catalysis on water splitting reaction by graphitic carbon supported NiO, NiS and NiSe nanoparticles
引用次数: 0
Abstract
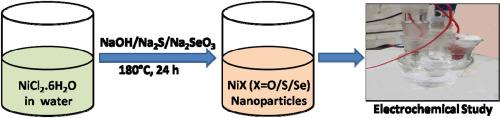
In this work, we have synthesized NiO, NiS and NiSe nanoparticles by similar hydrothermal method and the electrocatalytic activities of the graphite carbon-supported synthesized materials have been compared in reference to hydrogen and oxygen evolution reactions (HER and OER) in aqueous acidic and alkaline media respectively. The as-synthesized nanoparticles have been characterized by using powder X-ray diffraction, Fourier transform infrared spectroscopy, and scanning electron microscopic studies. The best electrocatalyst, NiSe provides a current density of 10 mA cm−2 at 259 mV overpotential for OER in 1.0 M KOH, which is superior to that of the state-of-the-art catalyst RuO2 in the same environment. For HER the best electrocatalyst, NiSe provides a current density of 10 mA cm−2 at 49.5 mV overpotential in 0.5 M H2SO4, which is again superior to Pt wire electrode. The order of electrocatalytic activity in both HER and OER has been found to follow the sequence: NiSe > NiS > NiO under the same electrochemical conditions, as have been evident from cyclic voltammetry, chronoamperometry and electrochemical impedance spectroscopic studies. While the electrochemical surface area is increased by 16.4 % and 37.3 % on changing the electrocatalyst from NiO to NiS and NiSe respectively, the chronoamperometric current densities are increased by 429 % and 635 % at 0.8 V for OER and 548 % and 9733 % at −0.4V for HER on changing the same materials. Thus, the enhancement in catalytic activity hangs mainly on the material characteristics besides the morphological improvement.
石墨碳支撑的 NiO、NiS 和 NiSe 纳米颗粒对水分离反应的双功能催化作用
在这项工作中,我们采用类似的水热法合成了 NiO、NiS 和 NiSe 纳米粒子,并比较了石墨碳支撑合成材料在酸性和碱性水介质中分别进行氢和氧进化反应(HER 和 OER)的电催化活性。通过粉末 X 射线衍射、傅立叶变换红外光谱和扫描电子显微镜研究对合成的纳米颗粒进行了表征。最佳电催化剂 NiSe 在 1.0 M KOH 中,过电位为 259 mV 时,OER 的电流密度为 10 mA cm-2,优于在相同环境中使用的最先进催化剂 RuO2。对于 HER 最佳电催化剂,NiSe 在 0.5 M H2SO4 中的过电位为 49.5 mV 时的电流密度为 10 mA cm-2,同样优于铂丝电极。研究发现,HER 和 OER 的电催化活性顺序如下在相同的电化学条件下,NiSe、NiS、NiO 的电催化活性顺序依次为:NiSe、NiS、NiO。将电催化剂从 NiO 改为 NiS 和 NiSe 后,电化学表面积分别增加了 16.4% 和 37.3%,而改变相同的材料后,OER 在 0.8V 时的时变电流密度分别增加了 429% 和 635%,HER 在-0.4V 时分别增加了 548% 和 9733%。因此,催化活性的提高主要取决于材料的特性,而不是形态的改善。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


