MOF-derived Ni3Fe/Ni/NiFe2O4@C for enhanced hydrogen storage performance of MgH2
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
Magnesium hydride (MgH2) is an important material for hydrogen (H2) storage and transportation owing to its high capacity and reversibility. However, its intrinsic properties have considerably limited its industrial application. In this study, the NiFe-800 catalyst as metal-organic framework (MOF) derivative was first utilized to promote the intrinsic properties of MgH2. Compared to pure MgH2, which releases 1.24 wt% H2 in 60 min at 275 °C, the MgH2-10 NiFe-800 composite releases 5.85 wt% H2 in the same time. Even at a lower temperature of 250 °C, the MgH2-10 NiFe-800 composite releases 3.57 wt% H2, surpassing the performance of pure MgH2 at 275 °C. Correspondingly, while pure MgH2 absorbs 2.08 wt% H2 in 60 min at 125 °C, the MgH2-10 NiFe-800 composite absorbs 5.35 wt% H2 in just 1 min. Remarkably, the MgH2-10 NiFe-800 composite absorbs 2.27 wt% H2 in 60 min at 50 °C and 4.64 wt% H2 at 75 °C. This indicates that MgH2-10 NiFe-800 exhibits optimum performance with excellent kinetics at low temperatures. Furthermore, the capacity of the MgH2-10 NiFe-800 composite remains largely stable after 10 cycles. Moreover, the Mg2Ni/Mg2NiH4 acts as a “hydrogen pump”, providing effective diffusion channels that enhance the kinetic process of the composite during cycling. Additionally, Fe0 facilitates electron transfer and creates hydrogen diffusion channels and catalytic sites. Finally, carbon (C) effectively prevents particle agglomeration and maintains the cyclic stability of the composites. Consequently, the synergistic effects of Mg2Ni/Mg2NiH4, Fe0, and C considerably improve the kinetic properties and cycling stability of MgH2. This work offers an effective and valuable approach to improving the hydrogen storage efficiency in the commercial application of MgH2.
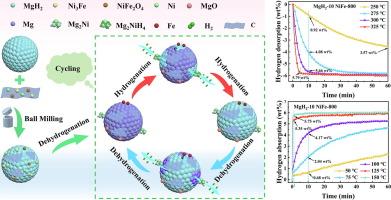
MOF 衍生的 Ni3Fe/Ni/NiFe2O4@C,用于提高 MgH2 的储氢性能
氢化镁(MgH2)具有高容量和可逆性,是一种重要的氢气(H2)储存和运输材料。然而,其固有特性在很大程度上限制了其工业应用。在本研究中,首先利用 NiFe-800 催化剂作为金属有机框架 (MOF) 衍生物来促进 MgH2 的内在特性。与纯 MgH2 在 275 °C 下 60 分钟内释放 1.24 wt% H2 相比,MgH2-10 NiFe-800 复合材料在相同时间内释放 5.85 wt% H2。即使在 250 °C 的较低温度下,MgH2-10 NiFe-800 复合材料也能释放出 3.57 wt% 的 H2,超过了纯 MgH2 在 275 °C 下的性能。相应地,纯 MgH2 在 125 °C 下 60 分钟内吸收了 2.08 wt% 的 H2,而 MgH2-10 NiFe-800 复合材料仅在 1 分钟内就吸收了 5.35 wt% 的 H2。值得注意的是,MgH2-10 NiFe-800 复合材料在 50 °C 下 60 分钟内吸收 2.27 wt% H2,在 75 °C 下吸收 4.64 wt% H2。这表明,MgH2-10 NiFe-800 在低温条件下表现出最佳性能和出色的动力学特性。此外,MgH2-10 NiFe-800 复合材料的容量在 10 次循环后基本保持稳定。此外,Mg2Ni/Mg2NiH4 起到了 "氢泵 "的作用,提供了有效的扩散通道,增强了复合材料在循环过程中的动力学过程。此外,Fe0 可促进电子转移,并创建氢扩散通道和催化位点。最后,碳(C)可有效防止颗粒团聚,保持复合材料的循环稳定性。因此,Mg2Ni/Mg2NiH4、Fe0 和 C 的协同作用大大改善了 MgH2 的动力学特性和循环稳定性。这项工作为提高 MgH2 商业应用中的储氢效率提供了一种有效而有价值的方法。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


