Stabilizing water and regulating interfacial electrostatic interaction with economical supporting salt for stable Zn metal anode
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
Developing rechargeable aqueous Zn batteries for large-scale energy storage is impeded by inadequate reversibility and stability of the Zn anode, primarily caused by parasitic reactions and heterogeneous deposition. This study proposes an economical electrolyte strategy to address these Zn-related issues. The addition of a supporting salt enhances the thermodynamic stability of water, reduces the number of highly reactive water molecules, and modulates the interfacial electrostatic interaction. This approach effectively suppresses hydrogen evolution reaction and uncontrolled deposition. Remarkably, the rationally proportioned electrolyte allows a high average Coulombic efficiency of 99.93% for 1000 cycles in a Zn||Cu battery and a prolonged lifespan exceeding 4800 h in Zn||Zn cells. The knock-on effect is that Zn||MnO2 pouch cells deliver stable cycling performance, demonstrating the viability of this approach for practical applications.
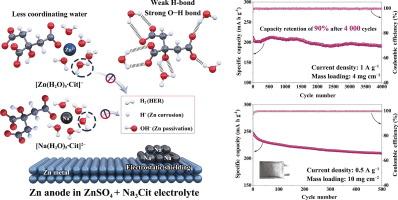
稳定水和调节与经济型支撑盐的界面静电相互作用,实现稳定的金属锌阳极
主要由寄生反应和异质沉积引起的锌阳极可逆性和稳定性不足阻碍了用于大规模储能的可充电锌水电池的开发。本研究提出了一种经济的电解质策略来解决这些与锌相关的问题。添加支撑盐可增强水的热力学稳定性,减少高活性水分子的数量,并调节界面静电相互作用。这种方法能有效抑制氢演化反应和不受控制的沉积。值得注意的是,合理配比的电解质使得锌||铜电池在 1000 次循环中的平均库仑效率高达 99.93%,并延长了锌||锌电池超过 4800 小时的寿命。其连锁效应是,Zn||MnO2 袋装电池具有稳定的循环性能,证明了这种方法在实际应用中的可行性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


