Understanding the iodine electrochemical behaviors in aqueous zinc batteries
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
Abstract
Iodine is widely used in aqueous zinc batteries (ZBs) due to its abundant resources, low cost, and active redox reactions. In addition to the active material in zinc-iodine batteries, iodine also plays an important role in other ZBs, such as regulating the electrochemical behavior of zinc ions, promoting the reaction kinetic and reversibility of other redox pairs, catalytic behaviors related to iodine reactions, coupling with other halogen ions, shuttle behaviors of polyiodides, etc. However, there is currently a lack of comprehensive discussion on these aspects. Here, this review provides a comprehensive overview of the electrochemical behaviors of iodide in the aqueous ZBs. The effect of iodine ions on the Zn2+ desolvation behaviors and the interfacial behaviors of Zn anode was summarized. Iodine redox pairs boosting other redox pairs, such as MnO2/Mn2+ redox pair and vanadium redox pair to obtain high reversibility and capacity was also discussed. Moreover, the catalytic behaviors related to iodine reactions in aqueous ZBs, synergistic reaction with other halogen ions and suppression of shuttle behaviors for high performance zinc-iodine batteries were systematically analyzed. Finally, future prospects for designing effective iodine electrochemical behaviors with practicability are proposed, which will provide scientific guidance for the practical application of iodine-related aqueous ZBs.
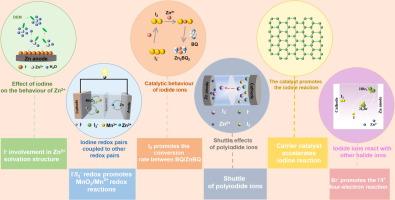
了解锌水电池中的碘电化学行为
碘因其资源丰富、成本低廉、氧化还原反应活跃而被广泛应用于水性锌电池(ZBs)中。除了作为锌碘电池的活性材料,碘在其他锌电池中也发挥着重要作用,如调节锌离子的电化学行为、促进其他氧化还原对的反应动力学和可逆性、与碘反应有关的催化行为、与其他卤素离子的偶联、聚碘化物的穿梭行为等。然而,目前还缺乏对这些方面的全面讨论。在此,本综述全面概述了碘化物在水性 ZBs 中的电化学行为。总结了碘离子对 Zn2+ 脱溶行为和 Zn 阳极界面行为的影响。还讨论了碘氧化还原对促进其他氧化还原对(如 MnO2/Mn2+ 氧化还原对和钒氧化还原对)以获得高可逆性和高容量的问题。此外,还系统分析了水性锌碘电池中与碘反应有关的催化行为、与其他卤素离子的协同反应以及抑制高性能锌碘电池的穿梭行为。最后,对设计具有实用性的有效碘电化学行为提出了展望,这将为碘相关水性锌碘电池的实际应用提供科学指导。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


