Effective degradation of phenol by in-situ photocatalytic-Fenton-like technology with BiVO4/Bi2WO6/Ti3C2 QDs
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Photocatalytically coupled Fenton-like is an environmentally promising technology for water treatment. In this work, BiVO4/Bi2WO6/Ti3C2 QDs composite photocatalysts (BBT) were prepared by hydrothermal and calcination methods to construct an in situ photocatalytic Fenton-like system. The photocatalytic degradation experiments were carried out under xenon lamp irradiation with phenol as the target pollutant. The BBT-2 photocatalyst could degrade 78.0% of phenol in 180 min. Compared with the catalyst without Ti3C2 QDs doping, the degradation efficiency was improved by 14%. The H2O2 yield of the BBT-2 catalyst reached 48.7 μM in the same time. Free radical trapping experiments showed that the BBT photocatalysts generated H2O2 by two-electron reduction of oxygen and hole oxidation of water. In addition, h+ and H2O2 play important roles in the phenol photocatalytic process during the degradation of phenol, and a possible reaction mechanism has been proposed. In this work, the combination of photocatalysis and Fenton-like reaction provides a solution for the green and sustainable degradation of highly toxic, difficult-to-degrade organic pollutants.
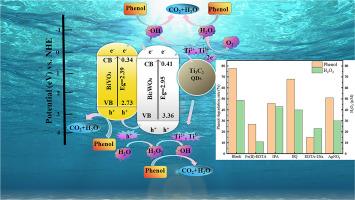
利用 BiVO4/Bi2WO6/Ti3C2 QDs 原位光催化-类芬顿技术有效降解苯酚
光催化耦合 Fenton-like 是一种具有环保前景的水处理技术。本研究采用水热法和煅烧法制备了 BiVO4/Bi2WO6/Ti3C2 QDs 复合光催化剂(BBT),构建了原位光催化 Fenton-like 系统。以苯酚为目标污染物,在氙灯照射下进行了光催化降解实验。BBT-2 光催化剂可在 180 分钟内降解 78.0% 的苯酚。与未掺杂 Ti3C2 QDs 的催化剂相比,降解效率提高了 14%。同时,BBT-2 催化剂的 H2O2 产率达到 48.7 μM。自由基捕获实验表明,BBT 光催化剂通过氧的双电子还原和水的空穴氧化产生 H2O2。此外,h+ 和 H2O2 在苯酚光催化降解过程中发挥了重要作用,并提出了可能的反应机理。在这项工作中,光催化与类 Fenton 反应的结合为高毒性、难降解有机污染物的绿色可持续降解提供了一种解决方案。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


