Novel synergistic mechanism of oxalic acid -CMC at the solid-liquid interface: For selective depression of talc from chalcopyrite
IF 8.3
2区 材料科学
Q1 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
Flotation separation of chalcopyrite and talc is challenging due to their surface hydrophobicity, requiring a selective depressant for talc to achieve high-quality copper concentrate. This study is the first to use oxalic acid (OA) and carboxymethyl cellulose (CMC) as combined depressants for talc, and investigated the depression mechanism through flotation tests and various analysis techniques. Mixed minerals-flotation results showed that with 60+60 mg/L OA+CMC, talc was strongly depressed (10.16% recovery) with slight chalcopyrite (86.54% recovery) impact. The selective depression effect of OA+CMC was further verified through actual ore flotation experiments. Contact angle and zeta potential test indicated that OA+CMC significantly increased the hydrophobicity difference between talc and chalcopyrite surfaces. Adsorption capacity and atomic force microscope(AFM) results further confirmed that OA promotes CMC adsorption on talc surface, forming a dense CMC layer, whereas CMC adsorption on chalcopyrite was relatively low. Scanning electron microscope energy spectrum spectroscopy (SEM-EDS), fourier transform infrared spectroscopy(FTIR), and molecular dynamics (MD) results indicate that the synergistic depression mechanism of OA and CMC involves the formation of multiple adsorption layers at the solid-liquid interface, leading to an increased presence of hydrophilic groups on the talc surface, which results in its depression.
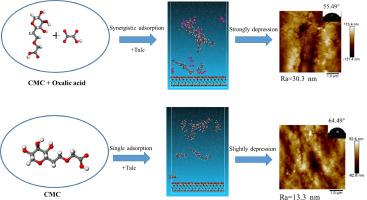
草酸-CMC 在固液界面上的新型协同机制:从黄铜矿中选择性去除滑石
由于黄铜矿和滑石的表面疏水性,它们的浮选分离具有挑战性,需要一种对滑石有选择性的抑制剂,以获得高质量的铜精矿。本研究首次使用草酸(OA)和羧甲基纤维素(CMC)作为滑石的联合抑制剂,并通过浮选试验和各种分析技术研究了抑制机制。混合矿物浮选结果表明,在 60+60 mg/L OA+CMC 的条件下,滑石受到强烈抑制(回收率为 10.16%),黄铜矿受到轻微影响(回收率为 86.54%)。实际矿石浮选实验进一步验证了 OA+CMC 的选择性抑制效果。接触角和 Zeta 电位测试表明,OA+CMC 显著增加了滑石和黄铜矿表面的疏水性差异。吸附容量和原子力显微镜(AFM)结果进一步证实,OA 能促进 CMC 在滑石表面的吸附,形成致密的 CMC 层,而 CMC 在黄铜矿上的吸附量相对较低。扫描电子显微镜能谱(SEM-EDS)、傅立叶变换红外光谱(FTIR)和分子动力学(MD)结果表明,OA 和 CMC 的协同抑制机制包括在固液界面形成多个吸附层,导致滑石表面亲水基团的增加,从而导致其抑制作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Applied Materials & Interfaces
工程技术-材料科学:综合
CiteScore
16.00
自引率
6.30%
发文量
4978
审稿时长
1.8 months
期刊介绍:
ACS Applied Materials & Interfaces is a leading interdisciplinary journal that brings together chemists, engineers, physicists, and biologists to explore the development and utilization of newly-discovered materials and interfacial processes for specific applications. Our journal has experienced remarkable growth since its establishment in 2009, both in terms of the number of articles published and the impact of the research showcased. We are proud to foster a truly global community, with the majority of published articles originating from outside the United States, reflecting the rapid growth of applied research worldwide.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


