The electrochemical synthesis of urea on triatomic cluster/Cu catalysts: A theoretical study
IF 5.7
2区 材料科学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Urea (NH2CONH2), a crucial nitrogen fertilizer and industrial raw material, is typically synthesized under rigorous reaction conditions. Currently, the electrocatalytic transformation of N2 and CO2 into urea is a promising strategy. However, finding a high-selectivity and high-activity catalyst remains a significant challenge. Herein, the activity of a series of transition metal clusters (VIII and IB groups) on copper-based catalysts for electrochemical coupling of CO2 and N2 has been systematically studied to produce urea via density functional theory (DFT). Most catalysts exhibit good thermodynamic stability and accomplish co-adsorb CO2 and N2. Notably, Fe3 and Ni3/Cu100 catalysts achieve C-N coupling via *CO and *N2, whereas Ru3, Rh3, Os3, and Ir3/Cu100 catalysts accomplish C-N coupling via *CO and *NHNH. Among all catalysts, the Ni3/Cu100 catalyst features excellent catalytic activity with a rate-determining step as low as 0.480 eV, and its C-N coupling only needs to overcome a barrier of 0.844 eV. Additionally, the Ni3/Cu100 catalyst can effectively inhibit the hydrogen evolution reaction (HER), further protonation of *CO and ammonia formation, thereby ensuring high selectivity for urea. Electronic structures analysis further reveals an “acceptance-donation” mechanism for the activation of *CO2 and *N2, with the introduction of the Ni3 cluster showing a decisive role. Therefore, this study may establish the foundation for the electrochemical synthesis of urea.
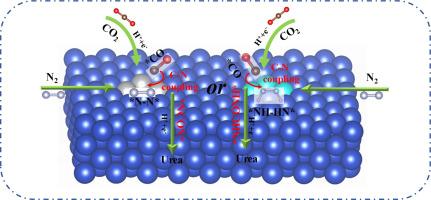
在三原子团簇/铜催化剂上电化学合成尿素:理论研究
尿素(NH2CONH2)是一种重要的氮肥和工业原料,通常在严格的反应条件下合成。目前,通过电催化将 N2 和 CO2 转化为尿素是一种很有前景的策略。然而,寻找一种高选择性和高活性催化剂仍是一项重大挑战。在此,我们通过密度泛函理论(DFT)系统地研究了铜基催化剂上的一系列过渡金属团簇(VIII 和 IB 基团)在 CO2 和 N2 电化学偶联生成尿素过程中的活性。大多数催化剂都表现出良好的热力学稳定性,并能完成 CO2 和 N2 的共吸附。值得注意的是,Fe3 和 Ni3/Cu100 催化剂通过 *CO 和 *N2 实现 C-N 偶联,而 Ru3、Rh3、Os3 和 Ir3/Cu100 催化剂则通过 *CO 和 *NHNH 实现 C-N 偶联。在所有催化剂中,Ni3/Cu100 催化剂具有出色的催化活性,其速率决定阶跃低至 0.480 eV,其 C-N 偶联只需克服 0.844 eV 的势垒。此外,Ni3/Cu100 催化剂还能有效抑制氢进化反应(HER)、*CO 的进一步质子化和氨的生成,从而确保尿素的高选择性。电子结构分析进一步揭示了*CO2 和*N2 活化的 "接受-捐献 "机制,其中 Ni3 团簇的引入起了决定性作用。因此,这项研究可为尿素的电化学合成奠定基础。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Surfaces and Interfaces
Chemistry-General Chemistry
CiteScore
8.50
自引率
6.50%
发文量
753
审稿时长
35 days
期刊介绍:
The aim of the journal is to provide a respectful outlet for ''sound science'' papers in all research areas on surfaces and interfaces. We define sound science papers as papers that describe new and well-executed research, but that do not necessarily provide brand new insights or are merely a description of research results.
Surfaces and Interfaces publishes research papers in all fields of surface science which may not always find the right home on first submission to our Elsevier sister journals (Applied Surface, Surface and Coatings Technology, Thin Solid Films)
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


