Clinical Benefits of new Systemic Therapy for Small-Cell Lung Cancer Over Two Decades: A Cross-Sectional Study
Abstract
Introduction
Small cell lung cancer (SCLC) is one of the most lethal malignancies worldwide. This study aimed to examine the clinical benefits of new systemic therapies derived from randomized controlled trials (RCTs) published from 2002 to 2023 based on the magnitude of clinical benefit scale developed by the European Society for Medical Oncology (ESMO-MCBS).
Methods
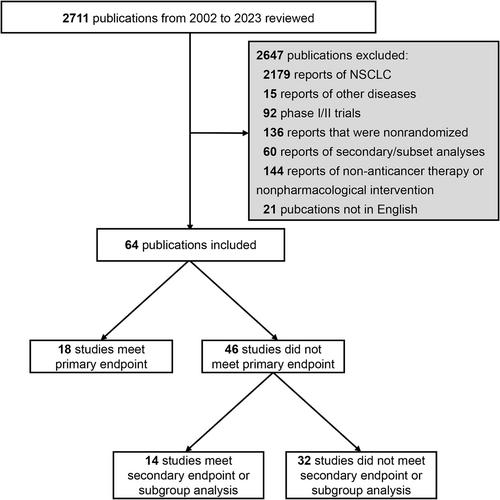
We searched PubMed for Phase 3 RCTs on systemic therapy for SCLC published between January 2002 and December 2023. Therapeutic benefit was graded from 5 to 1 according to the ESMO-MCBS framework, with a score of 4 or 5 representing a meaningful clinical benefit. The statistical power of the trial design was also assessed using ESMO-MCBS.
Results
Sixty-four RCTs with 23 683 participants were eligible for inclusion. The number of RCTs related to molecular targeted therapy or immunotherapy has increased over the years. Among the 62 RCTs for which statistical power could be evaluated, 38 (61.3%) were designed to identify an effect size that would meet the ESMO-MCBS benefit threshold and were less likely to investigate second- or subsequent-line treatment (15.8% vs. 50.0%, p = 0.004), have noninferiority design (0% vs. 25.0%, p = 0.002) and set PFS (0% vs. 16.7%) or response rate (0% vs. 16.7%) as the only primary endpoint (p = 0.002). The ESMO-MCBS framework was applied in 29 RCTs reporting positive results, and only 8 (27.6%) met the threshold for a clinical benefit. The RCTs designed to detect differences that would meet the thresholds were more likely to demonstrate meaningful clinical benefit (87.5% vs. 50.0%, p = 0.099).
Conclusion
Most positive SCLC-RCTs did not meet the ESMO-MCBS threshold for meaningful clinical benefits. Strict power calculations should be adopted in the design of future RCTs.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


