PCSK9 Inhibitors: The Evolving Future
Abstract
Introduction
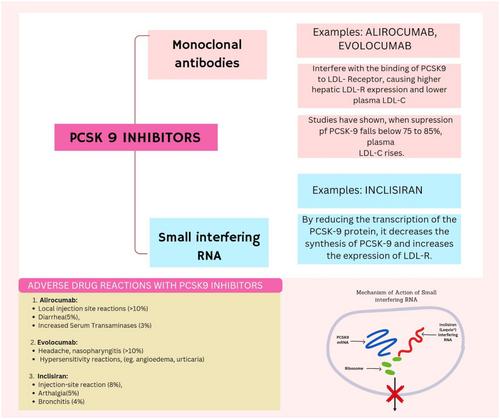
PCSK9 inhibitors are a novel class of medications that lower LDL cholesterol (LDL-C) by increasing LDL receptor activity, promoting clearance of LDL-C from the bloodstream. Over the years, PCSK9 inhibitors have been explored as adjunct therapies to statins or as monotherapy in high-risk cardiovascular patients.
Aim
This review aims to provide an updated perspective on PCSK9 inhibitors, assessing their clinical efficacy, safety, and significance, especially in light of recent clinical trials.
Methods
The review examines the role of PCSK9 in cholesterol regulation and summarizes the results of major cardiovascular trials, including FOURIER, SPIRE-1, SPIRE-2, and ODYSSEY Outcomes. It also discusses emerging treatments like small interfering RNA (siRNA) therapies and evaluates PCSK9 inhibitor effects on LDL-C and lipoprotein(a) levels.
Results
Clinical trials have shown PCSK9 inhibitors reduce LDL-C by up to 60%. In the FOURIER trial, evolocumab reduced LDL-C by 59% and major cardiovascular events by 15%–20%. The SPIRE-2 trial, despite early termination, showed a 21% risk reduction in the primary composite endpoint with bococizumab. The ODYSSEY Outcomes trial reported a 57% LDL-C reduction with alirocumab, alongside a 15% reduction in adverse events. Emerging treatments like Inclisiran offer long-term LDL-C control with fewer doses. PCSK9 inhibitors are generally well-tolerated, with the most common side effect being injection site reactions.
Conclusion
PCSK9 inhibitors significantly lower LDL-C and reduce cardiovascular events, offering promising therapies for high-risk patients, including those with familial hypercholesterolemia (FH) and those who cannot tolerate statins. Future research will focus on optimizing these inhibitors, integrating complementary therapies, and exploring gene-editing technologies to improve patient outcomes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


