Hybridized and engineered microbe for catalytic generation of peroxynitrite and cancer immunotherapy under sonopiezo initiation
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
Living therapeutics is an emerging antitumor modality by living microorganisms capable of selective tropism and effective therapeutics. Nevertheless, primitive microbes could only present limited therapeutic functionalities against tumors. Hybridization of the microbes with multifunctional nanocatalysts is of great significance to achieve enhanced tumor catalytic therapy. In the present work, nitric oxide synthase (NOS)–engineered Escherichia coli strain MG1655 (NOBac) was used to hybridize with the sonopiezocatalytic BaTiO3 nanoparticles (BTO NPs) for efficient tumor-targeted accumulation and antitumor therapy. Under ultrasound irradiation, superoxide anions created by the piezocatalytic reaction of BTO NPs could immediately react with nitric oxide (NO) generated from NOBac to produce highly oxidative peroxynitrite ONOO− species in cascade, resulting in robust tumor piezocatalytic therapeutic efficacy, prompting prominent and sustained antitumoral immunoactivation simultaneously. The present work presents a promising cancer immunotherapy based on the engineered and hybridized microbes for highly selective and sonopiezo-controllable tumor catalytic therapy.
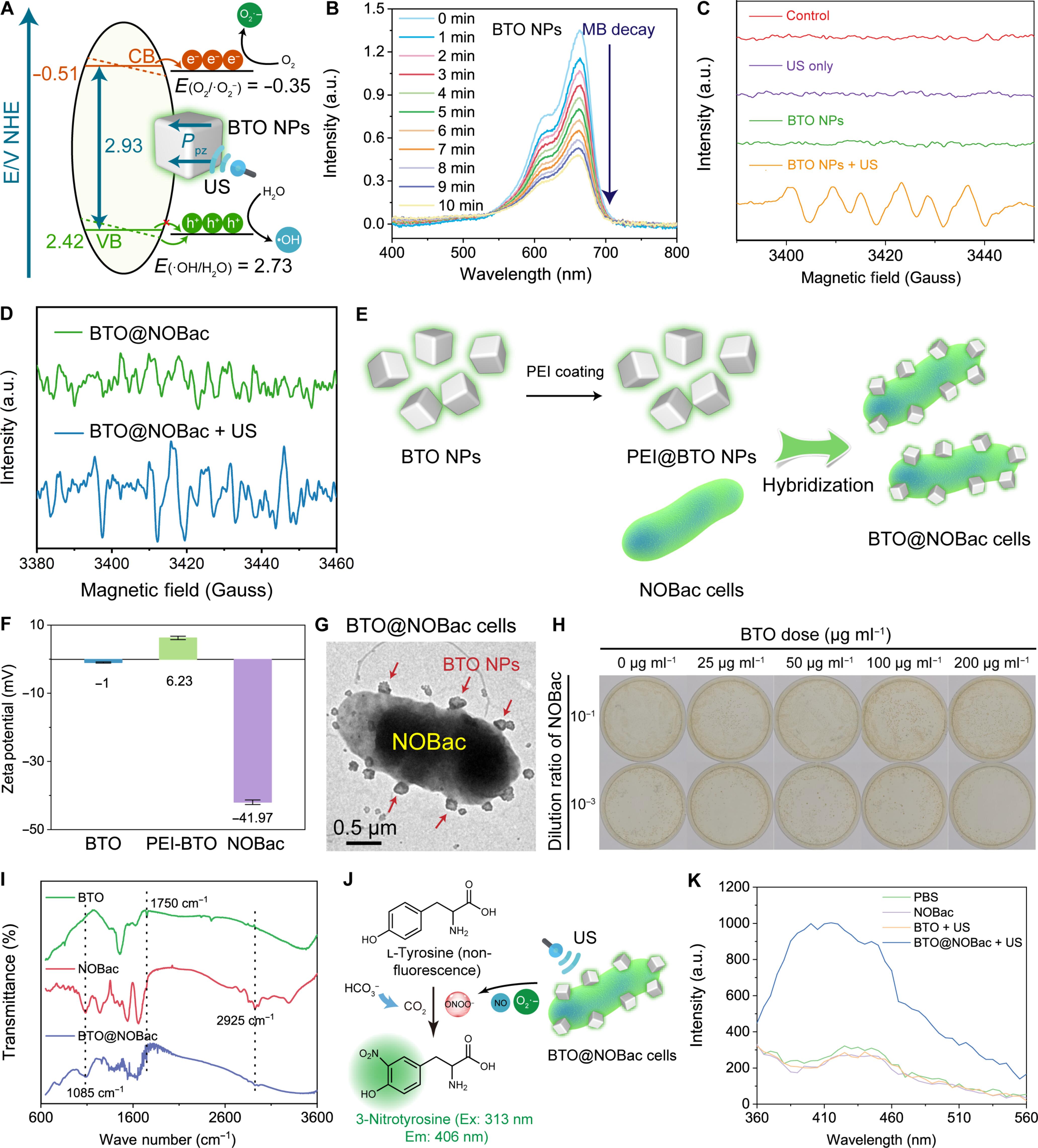
杂交和工程微生物,用于催化产生过氧化亚硝酸盐和在超声波启动下进行癌症免疫治疗。
活体疗法是一种新兴的抗肿瘤模式,由能够选择性滋养和有效治疗的活体微生物组成。然而,原始微生物只能提供有限的抗肿瘤治疗功能。将微生物与多功能纳米催化剂杂交,对实现更强的肿瘤催化治疗具有重要意义。在本研究中,一氧化氮合酶(NOS)工程化大肠杆菌菌株MG1655(NOBac)与超声波催化BaTiO3纳米粒子(BTO NPs)杂交,实现了高效的肿瘤靶向积累和抗肿瘤治疗。在超声辐照下,BTO NPs 压电催化反应产生的超氧阴离子可立即与 NOBac 产生的一氧化氮(NO)发生反应,级联产生高氧化性的过氧化亚硝酸盐 ONOO- 物种,从而产生强大的肿瘤压电催化疗效,同时促使抗肿瘤免疫激活作用显著而持久。本研究提出了一种基于工程化和杂交微生物的癌症免疫疗法,可用于高选择性和可控的肿瘤催化治疗。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


