A natural killer cell mimic against intracellular pathogen infections
IF 11.7
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
In the competition between the pathogen infection and the host defense, infectious microorganisms may enter the host cells by evading host defense mechanisms and use the intracellular biomolecules as replication nutrient. Among them, intracellular Staphylococcus aureus relies on the host cells to protect itself from the attacks by antibiotics or immune system to achieve long-term colonization in the host, and the consequent clinical therapeutic failures and relapses after antibiotic treatment. Here, we demonstrate that intracellular S. aureus surviving well even in the presence of vancomycin can be effectively eliminated using an emerging cell-mimicking therapeutic strategy. These cell mimics with natural killer cell–like activity (NKMs) are composed of a redox-responsive degradable carrier, and perforin and granzyme B within the carrier. NKMs perform far more effectivly than clinical antibiotics in treating intracellular bacterial infections, providing a direct evidence of the NK cell–mimicking immune mechanism in the treatment of intracellular S. aureus.
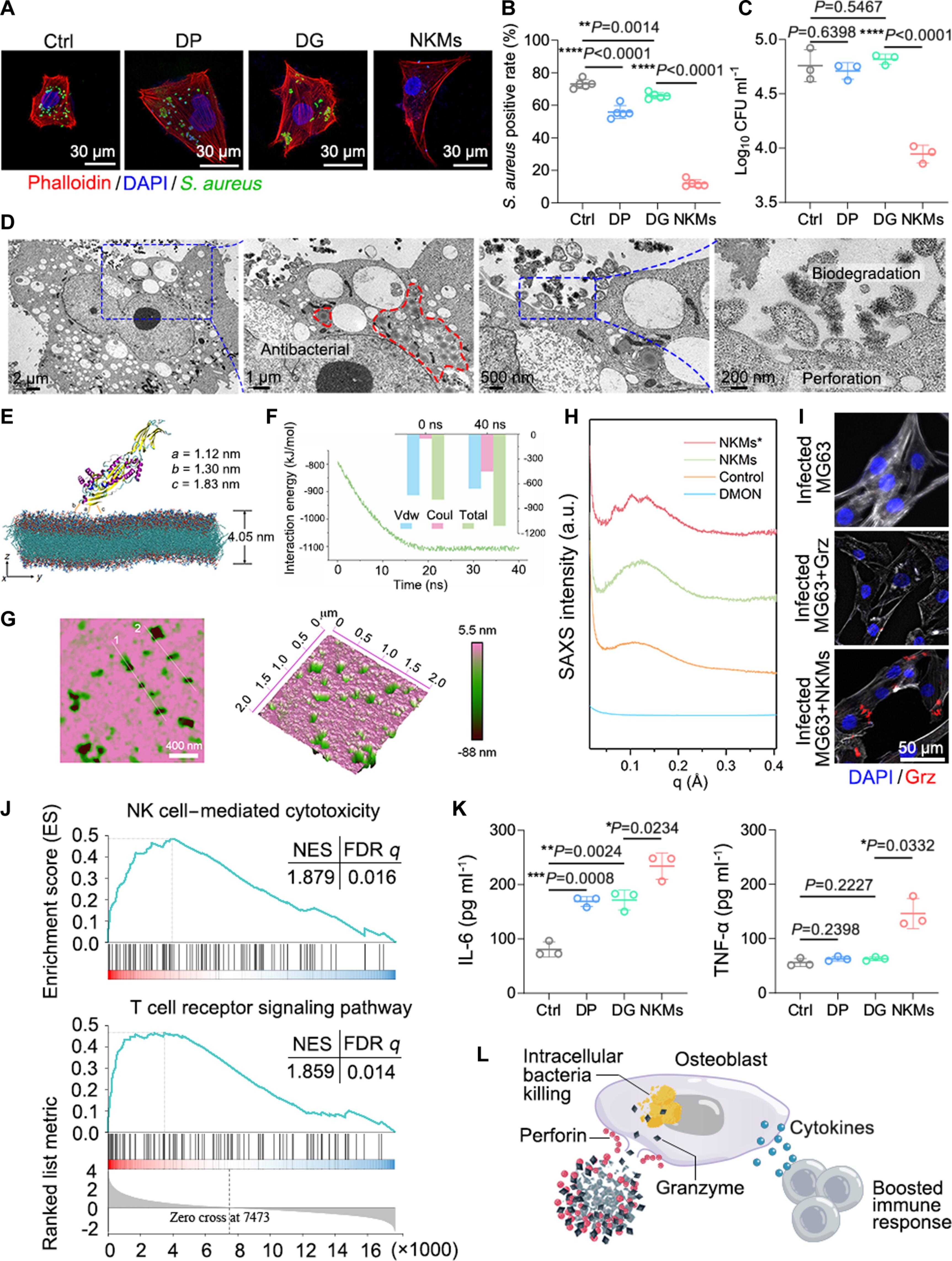
一种抗细胞内病原体感染的自然杀伤细胞模拟物。
在病原体感染与宿主防御的竞争中,感染性微生物可能通过逃避宿主防御机制进入宿主细胞,并利用细胞内的生物大分子作为复制养分。其中,细胞内金黄色葡萄球菌依靠宿主细胞保护自身免受抗生素或免疫系统的攻击,从而实现在宿主体内的长期定植,并因此导致临床治疗失败和抗生素治疗后复发。在这里,我们证明了即使在万古霉素的作用下也能很好存活的金黄色葡萄球菌,可以通过一种新兴的细胞模拟治疗策略有效地消灭。这些具有类似自然杀伤细胞活性的细胞模拟物(NKMs)由氧化还原反应可降解载体以及载体内的穿孔素和颗粒酶 B 组成。在治疗细胞内细菌感染方面,NKMs 的效果远远优于临床抗生素,直接证明了 NK 细胞模拟免疫机制在治疗细胞内金黄色葡萄球菌方面的作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Science Advances
综合性期刊-综合性期刊
CiteScore
21.40
自引率
1.50%
发文量
1937
审稿时长
29 weeks
期刊介绍:
Science Advances, an open-access journal by AAAS, publishes impactful research in diverse scientific areas. It aims for fair, fast, and expert peer review, providing freely accessible research to readers. Led by distinguished scientists, the journal supports AAAS's mission by extending Science magazine's capacity to identify and promote significant advances. Evolving digital publishing technologies play a crucial role in advancing AAAS's global mission for science communication and benefitting humankind.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


