Metabolomics in atrial fibrillation - A review and meta-analysis of blood, tissue and animal models
IF 4.9
2区 医学
Q1 CARDIAC & CARDIOVASCULAR SYSTEMS
引用次数: 0
Abstract
Background
Atrial fibrillation (AF) is a highly prevalent cardiac arrhythmia associated with severe cardiovascular complications. AF presents a growing global challenge, however, current treatment strategies for AF do not address the underlying pathophysiology. To advance diagnosis and treatment of AF, a deeper understanding of AF root causes is needed. Metabolomics is a fast approach to identify, quantify and analyze metabolites in a given sample, such as human serum or atrial tissue. In the past two decades, metabolomics have enabled research on metabolite biomarkers to predict AF, metabolic features of AF, and testing metabolic mechanisms of AF in animal models. Due to the field's rapid evolution, the methods of AF metabolomics studies have not always been optimal. Metabolomics research has lacked standardization and requires expertise to face methodological challenges.
Purpose of the review
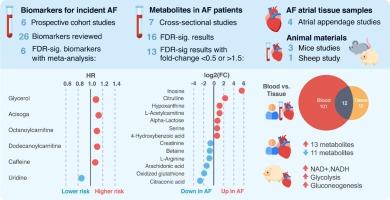
We summarize and meta-analyze metabolomics research on AF in human plasma and serum, atrial tissue, and animal models. We present the current progress on metabolic biomarkers candidates, metabolic features of clinical AF, and the translation of metabolomics findings from animal to human. We additionally discuss strengths and weaknesses of the metabolomics method and highlight opportunities for future AF metabolomics research.
心房颤动的代谢组学研究--血液、组织和动物模型的综述和荟萃分析。
背景:心房颤动(房颤)是一种与严重心血管并发症相关的高发心律失常。心房颤动是一项日益严峻的全球性挑战,然而,目前的心房颤动治疗策略并未解决其潜在的病理生理学问题。要推进心房颤动的诊断和治疗,需要深入了解心房颤动的根本原因。代谢组学是一种快速识别、量化和分析特定样本(如人血清或心房组织)中代谢物的方法。在过去的二十年里,代谢组学使人们能够研究预测房颤的代谢物生物标志物、房颤的代谢特征以及在动物模型中测试房颤的代谢机制。由于该领域发展迅速,房颤代谢组学研究的方法并不总是最佳的。代谢组学研究缺乏标准化,需要专业知识来应对方法学上的挑战:我们对人体血浆和血清、心房组织以及动物模型中有关房颤的代谢组学研究进行了总结和元分析。我们介绍了候选代谢生物标记物、临床房颤的代谢特征以及代谢组学研究结果从动物到人类的转化等方面的最新进展。此外,我们还讨论了代谢组学方法的优缺点,并强调了未来房颤代谢组学研究的机遇。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
10.70
自引率
0.00%
发文量
171
审稿时长
42 days
期刊介绍:
The Journal of Molecular and Cellular Cardiology publishes work advancing knowledge of the mechanisms responsible for both normal and diseased cardiovascular function. To this end papers are published in all relevant areas. These include (but are not limited to): structural biology; genetics; proteomics; morphology; stem cells; molecular biology; metabolism; biophysics; bioengineering; computational modeling and systems analysis; electrophysiology; pharmacology and physiology. Papers are encouraged with both basic and translational approaches. The journal is directed not only to basic scientists but also to clinical cardiologists who wish to follow the rapidly advancing frontiers of basic knowledge of the heart and circulation.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


