ER stress as a sentinel mechanism for ER Ca2+ homeostasis
IF 4.3
2区 生物学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Endoplasmic reticulum (ER) stress is triggered upon the interference with oxidative protein folding that aims to produce fully folded, disulfide-bonded and glycosylated proteins, which are then competent to exit the ER. Many of the enzymes catalyzing this process require the binding of Ca2+ ions, including the chaperones BiP/GRP78, calnexin and calreticulin. The induction of ER stress with a variety of drugs interferes with chaperone Ca2+ binding, increases cytosolic Ca2+through the opening of ER Ca2+ channels, and activates store-operated Ca2+ entry (SOCE). Posttranslational modifications (PTMs) of the ER Ca2+ handling proteins through ER stress-dependent phosphorylation or oxidation control these mechanisms, as demonstrated in the case of the sarco/endoplasmic reticulum ATPase (SERCA), inositol 1,4,5 trisphosphate receptors (IP3Rs) or stromal interaction molecule 1 (STIM1). Their aim is to restore ER Ca2+ homeostasis but also to increase Ca2+ transfer from the ER to mitochondria during ER stress. This latter function boosts ER bioenergetics, but also triggers apoptosis if ER Ca2+ signaling persists. ER Ca2+ toolkit oxidative modifications upon ER stress can occur within the ER lumen or in the adjacent cytosol. Enzymes involved in this redox control include ER oxidoreductin 1 (ERO1) or the thioredoxin-family protein disulfide isomerases (PDI) and ERp57. A tight, but adaptive connection between ER Ca2+ content, ER stress and mitochondrial readouts allows for the proper functioning of many tissues, including skeletal muscle, the liver, and the pancreas, where ER stress either maintains or compromises their function, depending on its extent and context. Upon mutation of key regulators of ER Ca2+ signaling, diseases such as muscular defects (e.g., from mutated selenoprotein N, SEPN1/SELENON), or diabetes (e.g., from mutated PERK) are the result.
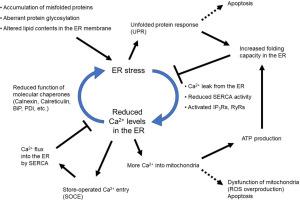
ER应激是ER Ca2+平衡的哨兵机制。
内质网(ER)应激是在氧化蛋白质折叠受到干扰时引发的,目的是产生完全折叠、二硫键结合和糖基化的蛋白质,然后使其能够离开ER。催化这一过程的许多酶都需要与 Ca2+ 离子结合,其中包括伴侣蛋白 BiP/GRP78、calnexin 和 calreticulin。用多种药物诱导ER应激会干扰伴侣的Ca2+结合,通过打开ER Ca2+通道增加细胞膜Ca2+,并激活贮存操作的Ca2+进入(SOCE)。ER钙离子处理蛋白的翻译后修饰(PTM)是通过ER应激依赖性磷酸化或氧化来控制这些机制的,如肌浆/内质网ATP酶(SERCA)、1,4,5-三磷酸肌醇受体(IP3Rs)或基质相互作用分子1(STIM1)的情况所示。它们的目的是恢复 ER Ca2+ 的平衡,同时在 ER 应激时增加 Ca2+ 从 ER 向线粒体的转移。后一种功能可增强 ER 的生物能,但如果 ER Ca2+ 信号持续存在,也会引发细胞凋亡。ER应激时ER Ca2+工具箱的氧化修饰可发生在ER腔内或邻近的细胞质中。参与这种氧化还原控制的酶包括ER氧化还原蛋白1(ERO1)或硫氧还原蛋白家族的蛋白二硫异构酶(PDI)和ERp57。ER Ca2+ 含量、ER 应激和线粒体读数之间存在着紧密的适应性联系,这使得包括骨骼肌、肝脏和胰腺在内的许多组织都能正常运作。一旦ER Ca2+信号的关键调节因子发生突变,就会导致肌肉缺陷(如硒蛋白N SEPN1/SELENON突变)或糖尿病(如PERK突变)等疾病。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Cell calcium
生物-细胞生物学
CiteScore
8.70
自引率
5.00%
发文量
115
审稿时长
35 days
期刊介绍:
Cell Calcium covers the field of calcium metabolism and signalling in living systems, from aspects including inorganic chemistry, physiology, molecular biology and pathology. Topic themes include:
Roles of calcium in regulating cellular events such as apoptosis, necrosis and organelle remodelling
Influence of calcium regulation in affecting health and disease outcomes
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


