Intermittent hypoxia-hyperoxia training ameliorates cognitive impairment and neuroinflammation in a rat model of Alzheimer’s disease
IF 2.7
4区 医学
Q3 NEUROSCIENCES
引用次数: 0
Abstract
Alzheimer’s disease (AD), characterized by severe and progressive cognitive decline, stands as one of the most prevalent and devastating forms of dementia. Based on our recent findings showing intermittent hypoxic conditioning improved neuronal function in patients with mild cognitive impairment, the present study aimed at investigating whether the neuroprotective effects of intermittent hypoxia can be replicated in a rat model of AD, which allows us to explore the underlying cellular mechanisms involving neuroinflammation, hypoxia inducible factor 1α (HIF1α), and cytochrome P450 family 2 subfamily E member 1 (CYP2E1). Forty-one adult male Wistar rats were randomly assigned to three groups: 1) Control group: received intracerebroventricular (ICV) injection of saline; 2) STZ group: received ICV injection of streptozotocin (STZ) to induce AD-like pathology; and 3) STZ + IHHT group received ICV injection of STZ as well as 15 daily sessions of intermittent hypoxia-hyperoxia training (IHHT). We observed that ICV injection of STZ inhibited spatial learning and memory in the rats assessed with Morris Water Maze test. The cognitive function declines were accompanied by increased expression of amyloid β peptide (Aβ), HIF1α, CYP2E1, and TNFα in hippocampus. Interestingly, IHHT significantly restored the STZ-induced cognitive dysfunction, while reduced expression of Aβ, CYP2E1, HIF1α and TNFα. We conclude that IHHT with mild hypoxia-hyperoxia can enhance spatial learning and memory and reduce the AD-like pathologic changes in rats. The neuroprotective outcome of IHHT may be related to anti-inflammatory effects in hippocampus.
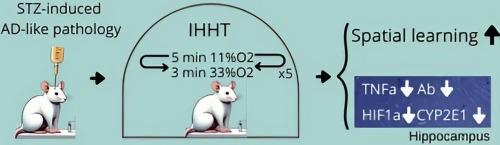
间歇性缺氧-过氧训练可改善阿尔茨海默病大鼠模型的认知障碍和神经炎症。
阿尔茨海默病(AD)以严重和进行性认知功能衰退为特征,是最常见和最具破坏性的痴呆症之一。基于我们最近的研究结果表明间歇性缺氧调节能改善轻度认知障碍患者的神经元功能,本研究旨在探讨间歇性缺氧的神经保护作用是否能在阿尔茨海默病大鼠模型中复制,从而探索涉及神经炎症、缺氧诱导因子1α(HIF1α)和细胞色素P450家族2亚家族E成员1(CYP2E1)的潜在细胞机制。将41只成年雄性Wistar大鼠随机分为三组:1)对照组:脑室内注射生理盐水;2)STZ组:脑室内注射链脲佐菌素(STZ)诱导AD样病理;3)STZ + IHHT组:脑室内注射STZ,同时每天进行15次间歇性缺氧-过氧训练(IHHT)。我们观察到,ICV注射STZ抑制了大鼠的空间学习和记忆能力。认知功能的下降伴随着海马中淀粉样β肽(Aβ)、HIF1α、CYP2E1和TNFα表达的增加。有趣的是,IHHT能明显恢复STZ诱导的认知功能障碍,同时降低Aβ、CYP2E1、HIF1α和TNFα的表达。我们的结论是,轻度缺氧-过氧的IHHT能增强大鼠的空间学习和记忆能力,减少AD样病理改变。IHHT的神经保护作用可能与海马的抗炎作用有关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Brain Research
医学-神经科学
CiteScore
5.90
自引率
3.40%
发文量
268
审稿时长
47 days
期刊介绍:
An international multidisciplinary journal devoted to fundamental research in the brain sciences.
Brain Research publishes papers reporting interdisciplinary investigations of nervous system structure and function that are of general interest to the international community of neuroscientists. As is evident from the journals name, its scope is broad, ranging from cellular and molecular studies through systems neuroscience, cognition and disease. Invited reviews are also published; suggestions for and inquiries about potential reviews are welcomed.
With the appearance of the final issue of the 2011 subscription, Vol. 67/1-2 (24 June 2011), Brain Research Reviews has ceased publication as a distinct journal separate from Brain Research. Review articles accepted for Brain Research are now published in that journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


