Inhibition of ferroptosis attenuate lipopolysaccharide-induced early pregnancy loss by protecting against decidual damage of stromal cells
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-28
DOI:10.1016/j.bbrc.2024.150904
引用次数: 0
Abstract
Endometrial decidualization is critical for successful embryo implantation. Dysregulation of the immune microenvironment can disrupt normal decidualization processes, potentially resulting in early pregnancy loss. Ferroptosis, a form of cell death dependent on iron and lipid hydroperoxides, is closely associated with inflammation. In this study, we developed an inflammatory early pregnancy loss model to elucidate the mechanisms of decidual damage induced by lipopolysaccharide (LPS) and to assess whether ferroptosis contributes to LPS-induced early pregnancy loss. Through in vivo experiments, we observed that embryo implantation was significantly inhibited and endometrial decidualization was impaired during LPS-induced early pregnancy loss. LPS exposure resulted in abnormal mitochondrial morphology, reduced antioxidant capacity, accumulation of reactive oxygen species (ROS) and disruptions in iron metabolism during decidualization in mouse endometrial stromal cells (mESCs). The administration of ferroptosis inhibitors, specifically ferrostatin-1 (Fer-1) and deferoxamine (DFO), effectively reversed embryo loss and mitigated the decidual damage associated with LPS-induced early pregnancy loss. Fer-1 and DFO exhibited resistance to ferroptosis during decidualization by modulating the antioxidant system and iron metabolism in mESCs, respectively. Our findings indicate that the inhibition of ferroptosis can confer protective effects against decidual damage during LPS-induced early pregnancy loss in mice.
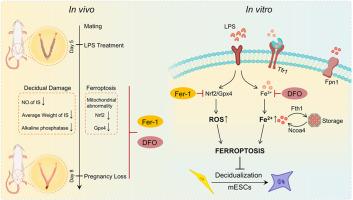
通过保护基质细胞免受蜕膜损伤,抑制铁变态反应可减轻脂多糖诱发的早期妊娠丢失。
子宫内膜蜕膜化是胚胎成功着床的关键。免疫微环境的失调会破坏正常的蜕膜化过程,从而可能导致早期妊娠失败。铁中毒是一种依赖于铁和脂质氢过氧化物的细胞死亡形式,与炎症密切相关。在这项研究中,我们建立了一个炎症性早期妊娠丢失模型,以阐明脂多糖(LPS)诱导的蜕膜损伤机制,并评估铁蜕变是否是 LPS 诱导的早期妊娠丢失的原因之一。通过体内实验,我们观察到在 LPS 诱导的早期妊娠丢失过程中,胚胎植入受到明显抑制,子宫内膜蜕膜化也受到损害。LPS暴露导致线粒体形态异常、抗氧化能力降低、活性氧(ROS)积累以及小鼠子宫内膜基质细胞(mESCs)蜕膜过程中铁代谢紊乱。服用铁蛋白沉积抑制剂,特别是铁前列素-1(Fer-1)和去铁胺(DFO),可有效逆转胚胎损失并减轻与 LPS 诱导的早期妊娠损失相关的蜕膜损伤。Fer-1和DFO分别通过调节mESCs的抗氧化系统和铁代谢,在蜕膜化过程中表现出抗铁蛋白沉积的能力。我们的研究结果表明,在LPS诱导的小鼠早期妊娠损失过程中,抑制铁蜕变可对蜕膜损伤产生保护作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


