Hemoglobin targeting potential of aminocarb pesticide: Investigation into dynamics, conformational stability, and energetics in solvent environment
IF 2.5
3区 生物学
Q3 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochemical and biophysical research communications
Pub Date : 2024-10-24
DOI:10.1016/j.bbrc.2024.150896
引用次数: 0
Abstract
Aminocarb (AMC), a carbamate pesticide, due to its prevalent usage exhibits increased accumulation in the environment affecting both insects and humans. It enters the human body via food grains and be transported through bloodstream. AMC's chemical structure, containing specific molecular frameworks and functional groups, enables it to bind with proteins like albumin and hemoglobin. Given that molecules with similar architecture are known to bind with hemoglobin, we aimed to explore Aminocarb's binding capability and the potential mechanism or mode of its interaction with hemoglobin. Hb being a tetramer with a profound interface between amino acid chains offers multiple binding sites. It is therefore important to investigate the structural aspects of binding of AMC by employing various spectroscopic and in-silico methods. The surface of the α1 chain near the α1β2 interface emerges as the preferred binding site for AMC, primarily due to its conformational restrictions. In its bound state, AMC tends to maintain a relaxed conformation, closely resembling its globally optimized geometry, and resides in close proximity to the α1 chain via multiple hydrophobic contacts and water bridge as observed in molecular dynamics (MD) simulations. Fluorescence quenching experiments showed moderate binding strength (7.7 × 10⁴ L M⁻1 at 288 K, 7.8 × 10⁴ L M⁻1 at 298 K, 7.9 × 10⁴ L M⁻1 at 308 K) and spontaneous binding, driven by hydrophobic and van der Waals interactions, as indicated by enthalpy (0.80–0.91 kJ mol⁻1), entropy (0.0970–0.0974 kJ mol⁻1), and Gibbs free energy (−27.13 to - 29.08 kJ mol⁻1). Circular dichroism experiments reveal no major structural changes in Hb. Quantum chemical calculations and MD simulations reveal conformation-dependent energy differences, enhancing our understanding of AMC's binding mechanism to Hb.
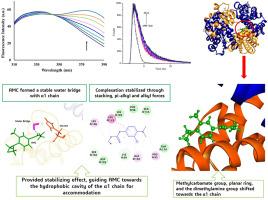
氨基甲酸乙酯农药的血红蛋白靶向潜力:溶剂环境中的动力学、构象稳定性和能量学研究。
氨灭威(AMC)是一种氨基甲酸酯杀虫剂,由于其广泛使用,在环境中的累积量越来越大,对昆虫和人类都有影响。它通过粮食进入人体,并通过血液传播。AMC 的化学结构包含特定的分子框架和功能基团,使其能够与白蛋白和血红蛋白等蛋白质结合。鉴于已知具有类似结构的分子可与血红蛋白结合,我们旨在探索氨灭威的结合能力及其与血红蛋白相互作用的潜在机制或模式。血红蛋白是一种四聚体,其氨基酸链之间的界面很深,可提供多个结合位点。因此,通过采用各种光谱和室内方法来研究 AMC 的结合结构非常重要。α1链靠近α1β2界面的表面成为AMC的首选结合位点,这主要是由于其构象限制。分子动力学(MD)模拟观察到,在结合态下,AMC倾向于保持松弛的构象,与其全局优化的几何形状非常相似,并通过多个疏水接触和水桥与α1链紧密结合。荧光淬灭实验显示其结合强度适中(288 K 时为 7.7 × 10⁴ L M-1,298 K 时为 7.8 × 10⁴ L M-1,7.焓(0.80-0.91 kJ mol-1)、熵(0.0970-0.0974 kJ mol-1)和吉布斯自由能(-27.13 至 - 29.08 kJ mol-1)表明,它们在疏水和范德华相互作用的驱动下自发结合。)环二色性实验显示 Hb 的结构没有发生重大变化。量子化学计算和 MD 模拟揭示了依赖于构象的能量差异,加深了我们对 AMC 与 Hb 结合机制的理解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
6.10
自引率
0.00%
发文量
1400
审稿时长
14 days
期刊介绍:
Biochemical and Biophysical Research Communications is the premier international journal devoted to the very rapid dissemination of timely and significant experimental results in diverse fields of biological research. The development of the "Breakthroughs and Views" section brings the minireview format to the journal, and issues often contain collections of special interest manuscripts. BBRC is published weekly (52 issues/year).Research Areas now include: Biochemistry; biophysics; cell biology; developmental biology; immunology
; molecular biology; neurobiology; plant biology and proteomics

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


