Triflic acid-promoted post-Ugi condensation for the assembly of 2,6-diarylmorpholin-3-ones†
IF 2.7
3区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
We report a two-step one-pot synthesis of the 2,6-diarylmorpholin-3-one core based on the Ugi reaction of 2-oxoaldehyde with 2-hydroxycarboxylic acid, a primary amine and tert-butyl isocyanide followed by a triflic acid-promoted intramolecular condensation accompanied by the loss of the isocyanide-originated amide moiety. The overall transformation proceeds with complete retention of stereoconfiguration at the 2-hydroxycarboxylic acid-derived chiral center, allowing the target morpholin-3-ones to be obtained in an enantiopure form. Subsequent double bond hydrogenation and amide reduction allow the degree of unsaturation to be reduced, providing a convenient entry to the cis-2,6-diphenylmorpholine motif.
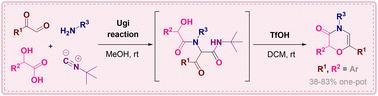
三氟丙烯酸促进乌基后缩合,用于 2,6-二芳基吗啉-3-酮的组装。
我们报告了一种 2,6-二芳基吗啉-3-酮核心的两步一步法合成方法,该方法基于 2-氧代醛与 2-羟基羧酸、伯胺和异氰酸叔丁酯的 Ugi 反应,随后在三氟酸的促进下进行分子内缩合,并伴随着异氰酸酰胺分子的损失。在整个转化过程中,2-羟基羧酸衍生的手性中心完全保留了立体构型,从而获得了对映纯的目标吗啉-3-酮。随后的双键氢化和酰胺还原使不饱和程度降低,从而方便地进入顺式-2,6-二苯基吗啉基团。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic & Biomolecular Chemistry
化学-有机化学
CiteScore
5.50
自引率
9.40%
发文量
1056
审稿时长
1.3 months
期刊介绍:
Organic & Biomolecular Chemistry is an international journal using integrated research in chemistry-organic chemistry. Founded in 2003 by the Royal Society of Chemistry, the journal is published in Semimonthly issues and has been indexed by SCIE, a leading international database. The journal focuses on the key research and cutting-edge progress in the field of chemistry-organic chemistry, publishes and reports the research results in this field in a timely manner, and is committed to becoming a window and platform for rapid academic exchanges among peers in this field. The journal's impact factor in 2023 is 2.9, and its CiteScore is 5.5.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


