A novel integrated testing strategy (ITS) for evaluating acute fish toxicity with new approach methodologies (NAMs)
IF 10.3
1区 环境科学与生态学
Q1 ENVIRONMENTAL SCIENCES
引用次数: 0
Abstract
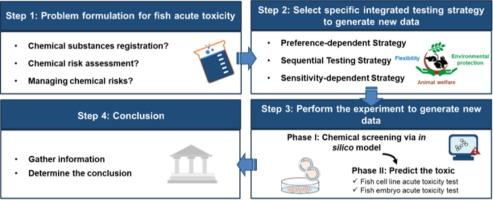
Acute fish toxicity (AFT) tests are performed in aquatic risk assessments of chemical compounds globally. However, the specific endpoint of in vivo AFT is based on the lethal concentration 50 (LC50), which is a serious challenge in terms of animal welfare. To support the 3Rs principle of replacing, reducing, and refining use of animals, integrated testing strategies (ITS) have recently been developed for environmental risk assessment. ITS efficiently integrates multiple types of information, especially new approach methodologies (NAMs), and further supports regulatory decision-making. Currently, an effective ITS framework for evaluating aquatic toxicity is lacking. Therefore, we aimed to develop a promising ITS for AFT using in silico, in vitro, and in vivo data. We established the ITS via in silico (OECD QSAR Toolbox 4.6), fish cell line acute toxicity (FCT), and fish embryo acute toxicity (FET) tests and then validated the NAMs with AFT testing. The NAM data were derived from the European Chemicals Agency (ECHA) dossier, toxicology databases, peer-reviewed research articles, and this study. For the first step in the ITS process, we aimed to design a high-throughput screening tool to identify non-toxic and toxic chemicals. We found that results of in silico, FCT, and FET tests alone were strongly correlated with AFT. Among the models, the in silico model was most suitable for identifying toxicants due to its high sensitivity and minimal animal use. Next, considering regulatory purposes and flexibility, we determined the predictive LC50 of toxic chemicals by pursuing a preference-dependent strategy, sequential testing strategy, and sensitivity-dependent strategy. All the strategies demonstrated a predictive power equal to or greater than 73%. In addition, to meet user preferences, our ITS approach has high flexibility and supports animal welfare and environmental protection. We have therefore developed multiple powerful, flexible, and more humane ITS methods for acute fish toxicity assessment by integrating NAMs.
利用新方法(NAMs)评估鱼类急性毒性的新型综合测试战略(ITS)
全球在对化合物进行水生风险评估时都会进行急性鱼类毒性(AFT)试验。然而,体内急性鱼类毒性试验的特定终点是以致死浓度 50(LC50)为基础的,这对动物福利是一个严峻的挑战。为了支持替代、减少和完善动物使用的 3R 原则,最近开发出了用于环境风险评估的综合测试策略(ITS)。ITS 可有效整合多种类型的信息,尤其是新方法(NAM),并进一步支持监管决策。目前,还缺乏一个有效的 ITS 框架来评估水生生物的毒性。因此,我们的目标是利用硅学、体外和体内数据,为 AFT 开发一种有前途的 ITS。我们通过硅学(OECD QSAR Toolbox 4.6)、鱼细胞系急性毒性(FCT)和鱼胚胎急性毒性(FET)测试建立了 ITS,然后用 AFT 测试验证了 NAM。NAM 数据来自欧洲化学品管理局 (ECHA) 档案、毒理学数据库、同行评审研究文章和本研究。在 ITS 流程的第一步,我们旨在设计一种高通量筛选工具来识别无毒和有毒化学品。我们发现,硅学、FCT 和 FET 单独测试的结果与 AFT 密切相关。在这些模型中,硅学模型因其灵敏度高、使用动物最少而最适合用于识别有毒物质。接下来,考虑到监管目的和灵活性,我们通过偏好依赖策略、顺序测试策略和灵敏度依赖策略确定了有毒化学物质的预测半致死浓度。所有策略的预测能力都等于或大于 73%。此外,为了满足用户的偏好,我们的 ITS 方法具有高度灵活性,并支持动物福利和环境保护。因此,我们开发了多种功能强大、灵活且更人性化的 ITS 方法,通过整合 NAMs 用于急性鱼类毒性评估。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Environment International
环境科学-环境科学
CiteScore
21.90
自引率
3.40%
发文量
734
审稿时长
2.8 months
期刊介绍:
Environmental Health publishes manuscripts focusing on critical aspects of environmental and occupational medicine, including studies in toxicology and epidemiology, to illuminate the human health implications of exposure to environmental hazards. The journal adopts an open-access model and practices open peer review.
It caters to scientists and practitioners across all environmental science domains, directly or indirectly impacting human health and well-being. With a commitment to enhancing the prevention of environmentally-related health risks, Environmental Health serves as a public health journal for the community and scientists engaged in matters of public health significance concerning the environment.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


