Leptin-activated hypothalamic BNC2 neurons acutely suppress food intake
IF 50.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
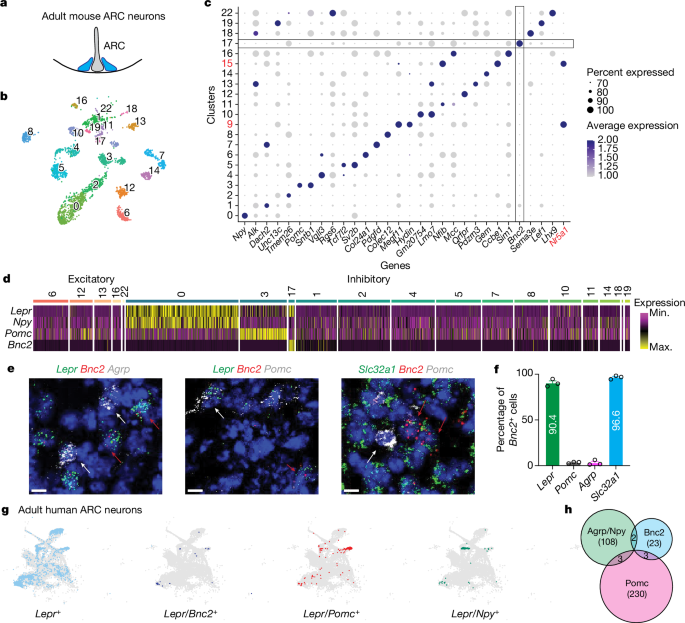
Leptin is an adipose tissue hormone that maintains homeostatic control of adipose tissue mass by regulating the activity of specific neural populations controlling appetite and metabolism1. Leptin regulates food intake by inhibiting orexigenic agouti-related protein (AGRP) neurons and activating anorexigenic pro-opiomelanocortin (POMC) neurons2. However, whereas AGRP neurons regulate food intake on a rapid time scale, acute activation of POMC neurons has only a minimal effect3–5. This has raised the possibility that there is a heretofore unidentified leptin-regulated neural population that rapidly suppresses appetite. Here we report the discovery of a new population of leptin-target neurons expressing basonuclin 2 (Bnc2) in the arcuate nucleus that acutely suppress appetite by directly inhibiting AGRP neurons. Opposite to the effect of AGRP activation, BNC2 neuronal activation elicited a place preference indicative of positive valence in hungry but not fed mice. The activity of BNC2 neurons is modulated by leptin, sensory food cues and nutritional status. Finally, deleting leptin receptors in BNC2 neurons caused marked hyperphagia and obesity, similar to that observed in a leptin receptor knockout in AGRP neurons. These data indicate that BNC2-expressing neurons are a key component of the neural circuit that maintains energy balance, thus filling an important gap in our understanding of the regulation of food intake and leptin action. We find that leptin-target neurons expressing basonuclin 2 in the arcuate nucleus that acutely suppress appetite by directly inhibiting agouti-related protein neurons are a key component of the neural circuit that maintains energy balance.

瘦素激活的下丘脑 BNC2 神经元能迅速抑制食物摄入量
瘦素是一种脂肪组织激素,它通过调节控制食欲和新陈代谢的特定神经群的活动来维持对脂肪组织质量的稳态控制1。瘦素通过抑制促食欲的激动相关蛋白(AGRP)神经元和激活促厌食的促胰皮质素(POMC)神经元来调节食物摄入量2。然而,AGRP 神经元能快速调节食物摄入量,而急性激活 POMC 神经元的效果却微乎其微3,4,5。这就提出了一种可能性,即存在一种迄今为止尚未发现的能快速抑制食欲的瘦素调控神经群。在这里,我们报告了在弓状核中发现的表达巴松肽2(Bnc2)的瘦素靶神经元新群体,它们通过直接抑制AGRP神经元来快速抑制食欲。与激活 AGRP 的效果相反,激活 BNC2 神经元会引起饥饿小鼠(而非喂食小鼠)的场所偏好,表明其具有积极的价值。BNC2 神经元的活性受瘦素、食物感觉线索和营养状况的调节。最后,删除 BNC2 神经元中的瘦素受体会导致明显的食欲亢进和肥胖,这与敲除 AGRP 神经元中的瘦素受体所观察到的情况相似。这些数据表明,BNC2表达神经元是维持能量平衡的神经回路的一个关键组成部分,从而填补了我们对食物摄入和瘦素作用调控认识的一个重要空白。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


