Rhythmic IL-17 production by γδ T cells maintains adipose de novo lipogenesis
IF 48.5
1区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
Abstract
The circadian rhythm of the immune system helps to protect against pathogens1–3; however, the role of circadian rhythms in immune homeostasis is less well understood. Innate T cells are tissue-resident lymphocytes with key roles in tissue homeostasis4–7. Here we use single-cell RNA sequencing, a molecular-clock reporter and genetic manipulations to show that innate IL-17-producing T cells—including γδ T cells, invariant natural killer T cells and mucosal-associated invariant T cells—are enriched for molecular-clock genes compared with their IFNγ-producing counterparts. We reveal that IL-17-producing γδ (γδ17) T cells, in particular, rely on the molecular clock to maintain adipose tissue homeostasis, and exhibit a robust circadian rhythm for RORγt and IL-17A across adipose depots, which peaks at night. In mice, loss of the molecular clock in the CD45 compartment (Bmal1∆Vav1) affects the production of IL-17 by adipose γδ17 T cells, but not cytokine production by αβ or IFNγ-producing γδ (γδIFNγ) T cells. Circadian IL-17 is essential for de novo lipogenesis in adipose tissue, and mice with an adipocyte-specific deficiency in IL-17 receptor C (IL-17RC) have defects in de novo lipogenesis. Whole-body metabolic analysis in vivo shows that Il17a−/−Il17f−/− mice (which lack expression of IL-17A and IL-17F) have defects in their circadian rhythm for de novo lipogenesis, which results in disruptions to their whole-body metabolic rhythm and core-body-temperature rhythm. This study identifies a crucial role for IL-17 in whole-body metabolic homeostasis and shows that de novo lipogenesis is a major target of IL-17. Innate IL-17-producing T cells—in particular, adipose γδ17 T cells—are enriched in molecular-clock genes, and the circadian expression of IL-17A and RORγt by these cells has a role in maintaining local homeostasis and regulating lipogenesis.
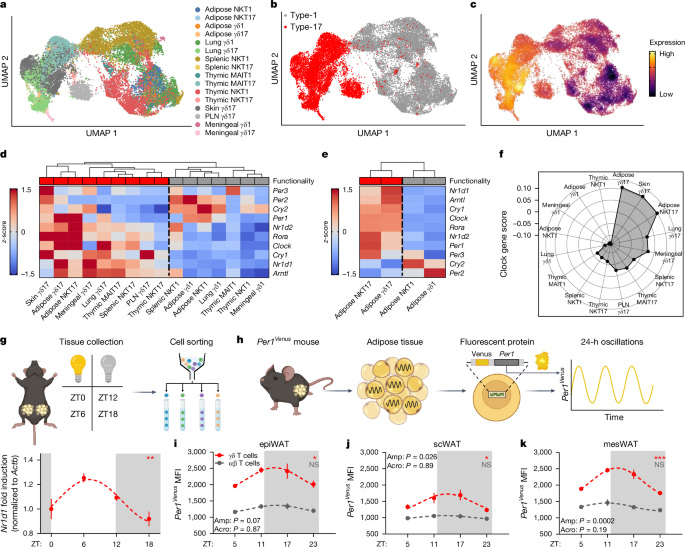

γδ T 细胞有节奏地产生 IL-17 可维持脂肪的新生脂质形成
免疫系统的昼夜节律有助于抵御病原体的侵袭1,2,3;然而,人们对昼夜节律在免疫平衡中的作用却不甚了解。先天性 T 细胞是组织驻留淋巴细胞,在组织稳态中起着关键作用4,5,6,7。在这里,我们利用单细胞 RNA 测序、分子钟报告基因和遗传操作来证明,与产生 IFNγ 的 T 细胞相比,产生 IL-17 的先天性 T 细胞(包括 γδ T 细胞、不变性自然杀伤 T 细胞和粘膜相关不变性 T 细胞)富含分子钟基因。我们发现,产生 IL-17 的 γδ (γδ17)T 细胞尤其依赖分子钟来维持脂肪组织的平衡,并在整个脂肪沉积中表现出 RORγt 和 IL-17A 强有力的昼夜节律,这种节律在夜间达到峰值。在小鼠中,CD45区(Bmal1∆Vav1)分子钟的缺失会影响脂肪γδ17 T细胞产生IL-17,但不会影响αβ或产生IFNγ的γδ(γδIFNγ)T细胞产生细胞因子。昼夜节律IL-17对脂肪组织中的新脂肪生成至关重要,脂肪细胞特异性缺乏IL-17受体C(IL-17RC)的小鼠在新脂肪生成方面存在缺陷。体内全身代谢分析表明,Il17a-/-Il17f-/-小鼠(缺乏IL-17A和IL-17F的表达)的新生脂肪生成昼夜节律存在缺陷,导致其全身代谢节律和核心体温节律紊乱。这项研究确定了 IL-17 在全身代谢平衡中的关键作用,并表明新脂肪生成是 IL-17 的一个主要靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature
综合性期刊-综合性期刊
CiteScore
90.00
自引率
1.20%
发文量
3652
审稿时长
3 months
期刊介绍:
Nature is a prestigious international journal that publishes peer-reviewed research in various scientific and technological fields. The selection of articles is based on criteria such as originality, importance, interdisciplinary relevance, timeliness, accessibility, elegance, and surprising conclusions. In addition to showcasing significant scientific advances, Nature delivers rapid, authoritative, insightful news, and interpretation of current and upcoming trends impacting science, scientists, and the broader public. The journal serves a dual purpose: firstly, to promptly share noteworthy scientific advances and foster discussions among scientists, and secondly, to ensure the swift dissemination of scientific results globally, emphasizing their significance for knowledge, culture, and daily life.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


