Selective Hydrolysis of Heterooligosaccharides by Poly(acrylate) Gel Catalysts
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Natural glycoside hydrolases are distinguished by their ability to hydrolyze glycosidic bonds with high efficiency and selectivity. This feature is achieved through specific interactions in the active site during catalytic turnover and is not just facilitated by two catalytically active amino acids. Intrigued by these features, a biomimetic α-galactosidase mimic was developed using an empirical catalyst design. Starting with a library of 704 gels of which 250 have a unique composition synthesized from TEGDMA cross-linker and 7 selected monomers, 238 monomodal gels are evaluated for their ability to hydrolyze the 1→6 α-glycosidic bond in the disaccharide melibiose. Among those, 13 polyacrylate gels with the potential for high catalytic activity are identified using spectrophotometric screening assays based on Schiff bases formed with toluidine. The best-performing polyacrylate (gel A) was found to have a 1500-fold higher proficiency to hydrolyze the 1→6 α-glycosidic bond in melibiose over the 1→2 α-glycosidic bond in sucrose, translating to selective hydrolysis of the glycosidic linkages in the trisaccharide raffinose. The matrix of gel A is composed of 25 mol % TEGDMA cross-linker and equimolar amounts of cyclohexyl, butyl, and benzyl acrylate accounting for CH-π and hydrophobic interaction in the surrounding of a hydrolytic binuclear Cu(II) complex. The combined observations underline a paramount influence of matrix-stabilizing effects on the transition state of the hydrolysis of glycosidic bonds and may pave the way for the rapid development of catalysts transforming biomass.
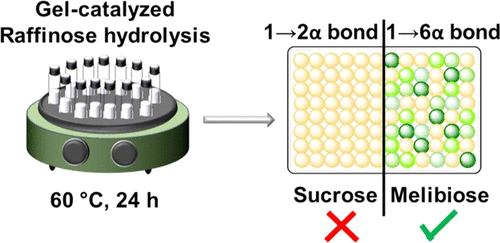
聚丙烯酸酯凝胶催化剂对杂环戊二糖的选择性水解作用
天然糖苷水解酶的与众不同之处在于它们能够高效率、高选择性地水解糖苷键。这一特点是在催化周转过程中通过活性位点的特定相互作用实现的,而不仅仅是由两个具有催化活性的氨基酸促成的。受这些特征的启发,我们利用经验催化剂设计开发了一种生物拟态 α-半乳糖苷酶模拟物。从一个包含 704 种凝胶(其中 250 种具有由 TEGDMA 交联剂和 7 种选定单体合成的独特成分)的库开始,对 238 种单模态凝胶水解二糖三聚糖中 1→6 α-糖苷键的能力进行了评估。利用基于与甲苯胺形成的希夫碱的分光光度筛选测定法,确定了其中 13 种具有高催化活性潜力的聚丙烯酸酯凝胶。研究发现,性能最好的聚丙烯酸酯(凝胶 A)水解三聚木糖中 1→6 α-糖苷键的能力比水解蔗糖中 1→2 α-糖苷键的能力高出 1500 倍,从而可选择性地水解三糖棉子糖中的糖苷键。凝胶 A 的基质由 25 mol % 的 TEGDMA 交联剂和等摩尔量的丙烯酸环己基酯、丙烯酸丁酯和丙烯酸苄酯组成,它们在水解双核 Cu(II) 复合物周围起着 CH-π 和疏水作用。综合观察结果凸显了基质稳定效应对水解糖苷键过渡状态的重要影响,并可能为快速开发转化生物质的催化剂铺平道路。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


