Brønsted Acid-Triggered Fast Synthesis Pathway of Furfural to Ethyl Levulinate by PtZn Supported on ZSM-5 Nanosheets
IF 11.3
1区 化学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
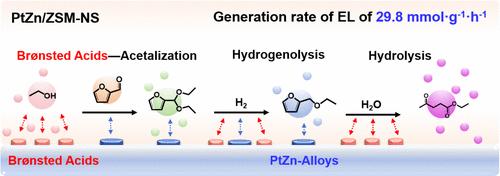
Furfural (FUR) is widely used to synthesize alkyl levulinate (AL), an important biomass-derived compound for industrial use. Traditional synthesis pathways, including hydrogenation, etherification, and hydrolysis, are slow due to high activation energy requirements. This study presents a pathway using ethyl levulinate (EL) as a model AL. The process starts with the acetalization of FUR to produce 2-(diethoxymethyl)furan (DEMF) using a Brønsted acid–based ZSM-5 nanosheet-supported PtZn (PtZn/ZSM-NS) catalyst. DEMF is then hydrogenolyzed to form 2-(ethoxymethyl)furan (EMF), which is hydrolyzed to produce EL at a rate of 29.8 mmol·g–1h–1, over 20 times faster than with a Lewis acid–based catalyst. In the initial step, Brønsted acid sites on the PtZn/ZSM-NS activate ethanol to generate an acetate-like intermediate (COOθ), which facilitates the acetalization of FUR to produce DEMF. This step is crucial for efficiently producing EL using the PtZn/ZSM-NS catalyst. Subsequently, EMF is easily formed through the hydrogenolysis of DEMF instead of through the etherification of furfuryl alcohol. Additionally, highly dispersed PtZn alloys on PtZn/ZSM-NS are essential for optimizing the adsorption strength, thereby accelerating the overall reaction. Using this pathway, the PtZn/ZSM-NS catalyst achieves an EL yield of up to 89.5 wt % at 200 °C in just 1 h.
以 ZSM-5 纳米片为载体的 PtZn 触发的糠醛到乙酰丙酸乙酯的快速合成途径
糠醛(FUR)被广泛用于合成左旋乙酸烷基酯(AL),这是一种重要的工业用生物质衍生化合物。传统的合成途径(包括氢化、醚化和水解)因需要较高的活化能而进展缓慢。本研究提出了一种以乙酰丙酮酸乙酯(EL)为 AL 模型的合成途径。该工艺首先使用基于布氏酸的 ZSM-5 纳米片支撑铂锌(PtZn/ZSM-NS)催化剂将 FUR 乙缩醛化,生成 2-(二乙氧基甲基)呋喃(DEMF)。然后,DEMF 被氢解生成 2-(乙氧基甲基)呋喃 (EMF),EMF 被水解生成 EL 的速度为 29.8 mmol-g-1h-1,比使用路易斯酸催化剂快 20 多倍。在初始步骤中,PtZn/ZSM-NS 上的布氏酸位点激活乙醇,生成类似乙酸酯的中间体 (COOθ),从而促进 FUR 的缩醛化,生成 DEMF。这一步骤对于使用 PtZn/ZSM-NS 催化剂高效生产 EL 至关重要。随后,通过 DEMF 的氢解而非糠醇的醚化,EMF 很容易生成。此外,PtZn/ZSM-NS 上高度分散的 PtZn 合金对于优化吸附强度,从而加速整个反应至关重要。利用这种途径,PtZn/ZSM-NS 催化剂在 200 °C 温度下只需 1 小时就能获得高达 89.5 wt % 的 EL 产率。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

ACS Catalysis
CHEMISTRY, PHYSICAL-
CiteScore
20.80
自引率
6.20%
发文量
1253
审稿时长
1.5 months
期刊介绍:
ACS Catalysis is an esteemed journal that publishes original research in the fields of heterogeneous catalysis, molecular catalysis, and biocatalysis. It offers broad coverage across diverse areas such as life sciences, organometallics and synthesis, photochemistry and electrochemistry, drug discovery and synthesis, materials science, environmental protection, polymer discovery and synthesis, and energy and fuels.
The scope of the journal is to showcase innovative work in various aspects of catalysis. This includes new reactions and novel synthetic approaches utilizing known catalysts, the discovery or modification of new catalysts, elucidation of catalytic mechanisms through cutting-edge investigations, practical enhancements of existing processes, as well as conceptual advances in the field. Contributions to ACS Catalysis can encompass both experimental and theoretical research focused on catalytic molecules, macromolecules, and materials that exhibit catalytic turnover.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


