Design, synthesis, and in vitro and in vivo biological evaluation of dihydroartemisinin derivatives as potent anti-cancer agents with ferroptosis-inducing and apoptosis-activating properties
IF 5.9
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
Abstract
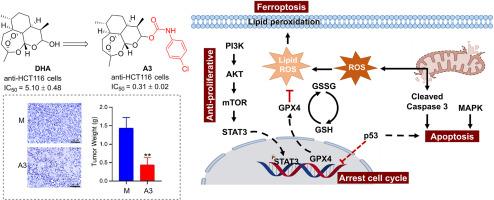
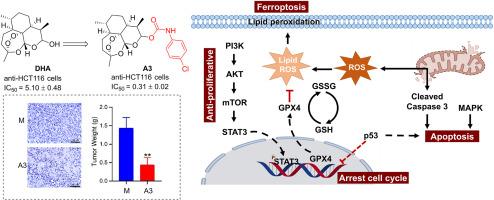
Natural products play a pivotal role in drug development, including their direct use as pharmaceuticals and their structural modification, yielding molecules with enhanced therapeutic potential. The discovery of bioactive molecules, lead compounds, and novel drugs is intrinsically linked to the structural optimization of natural products. In this study, forty-one derivatives of dihydroartemisinin (DHA) were synthesized by incorporating fragments with anti-tumour activity via molecular hybridization, and assessed for their anti-proliferative activity against human cancer cell lines (A549, Bel-7402, HCT-116, and SW620) and normal human liver cells (LO2). Most derivatives exhibited superior anti-proliferative activity compared to DHA. Notably, compound A3, featuring a 4-Cl phenyl carbamate moiety, demonstrated significant anti-proliferative activity against HCT-116 cells with an IC50 of 0.31 μM, making it 16-fold more potent than DHA (IC50 = 5.10 μM). The anti-proliferative mechanism did not involve cytotoxicity (SI = 54.13), indicating its superior safety profile compared to DHA (SI = 1.65).
Further mechanistic studies revealed that compound A3 inhibits HCT-116 cell proliferation by modulating the expression of PI3K/AKT/mTOR and STAT3 proteins. STAT3 downregulation represses the expression of the critical ferroptosis protein glutathione peroxidase 4 (GPX4), aggravating the accumulation of reactive oxygen species (ROS) and depletion of glutathione (GSH). This redox imbalance triggers and accelerates ferroptosis. Additionally, A3 also induces apoptosis by damaging mitochondria and influencing MAPK signaling. Compound A3 arrested cells in the G2/M phase by regulating p53 expression. In an HCT-116 xenograft mouse model, compound A3 exhibited significant anti-cancer efficacy, with a tumor growth inhibition rate of 58.7 %. Therefore, compound A3 thus has the potential to serve as a lead compound for the development of new anti-tumor drugs.
设计、合成二氢青蒿素衍生物并对其进行体内外生物学评价,使其成为具有铁蛋白沉降诱导和细胞凋亡激活特性的强效抗癌剂
天然产品在药物开发中发挥着举足轻重的作用,包括直接用作药物和对其进行结构改造,从而产生具有更大治疗潜力的分子。生物活性分子、先导化合物和新型药物的发现与天然产物的结构优化有着内在联系。在这项研究中,通过分子杂交将具有抗肿瘤活性的片段加入到双氢青蒿素(DHA)中,合成了 41 种衍生物,并评估了它们对人类癌细胞株(A549、Bel-7402、HCT-116 和 SW620)和正常人类肝细胞(LO2)的抗增殖活性。与 DHA 相比,大多数衍生物都表现出更强的抗增殖活性。值得注意的是,化合物 A3 具有 4-Cl 苯基氨基甲酸酯分子,对 HCT-116 细胞具有显著的抗增殖活性,IC50 为 0.31 μM,比 DHA(IC50 = 5.10 μM)强 16 倍。进一步的机理研究发现,化合物 A3 通过调节 PI3K/AKT/mTOR 和 STAT3 蛋白的表达来抑制 HCT-116 细胞的增殖。STAT3 的下调抑制了关键的铁氧化蛋白谷胱甘肽过氧化物酶 4(GPX4)的表达,加剧了活性氧(ROS)的积累和谷胱甘肽(GSH)的消耗。这种氧化还原失衡引发并加速了铁凋亡。此外,A3 还会通过破坏线粒体和影响 MAPK 信号转导来诱导细胞凋亡。化合物 A3 通过调节 p53 的表达,使细胞停滞在 G2/M 阶段。在 HCT-116 异种移植小鼠模型中,化合物 A3 表现出显著的抗癌效果,肿瘤生长抑制率达 58.7%。因此,化合物 A3 有潜力成为开发新型抗肿瘤药物的先导化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


