A two-pronged approach:Bulk-phase substitution and second-phase synergism to enhance the anion redox stability of layered oxide cathode for sodium-ion batteries
IF 17.1
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
As a supplement to lithium-ion batteries, sodium-ion batteries have great application potential in large-scale energy storage equipment, vehicle start-stop power supplies, and low-speed electric vehicle power. P3-NaxLiyMn1-yO2 (NLMO), has received widespread attention due to its higher charge voltage and discharge capacity. However, poor cycle stability and rapid voltage platform decay caused by the release of oxygen during charging hinder its practical application. This paper reports a new NLMO modification strategy, which effectively improves the cycling stability of NLMO by substituting Zr4+ for Mn4+ in the bulk phase to suppress the O2−/On− redox couple activity and stabilize the ZrO2 second phase lattice. Under the combined action of substitution and second phase, the capacity retention rate of [email protected] was 85.4 % after 100 cycles at a rate of 5 C. The results show that Zr4+ changes the coordination environment and electronic structure of O in the crystal lattice, prompting further splitting of Mn t2 g, thereby increasing the proportion of Mn 3d orbitals near the Fermi level. During the first discharge process, due to the charge balancing effect, Mn3+/Mn4+ replaced the electrochemical activity of O2−/On−, obtaining a specific capacity of up to 161 mAh g−1. This internal and external improvement idea provides a new direction for solving the poor cycle stability of NLMO high specific energy sodium-ion battery cathode materials and improving the specific capacity.
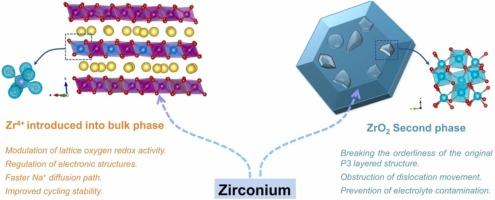
双管齐下:用体相置换和第二相协同作用增强钠离子电池层状氧化物阴极的阴离子氧化还原稳定性
作为锂离子电池的补充,钠离子电池在大型储能设备、汽车启停电源和低速电动汽车动力方面具有巨大的应用潜力。P3-NaxLiyMn1-yO2(NLMO)因其较高的充电电压和放电容量而受到广泛关注。然而,由于充电过程中氧气的释放导致循环稳定性差和电压平台快速衰减,阻碍了其实际应用。本文报道了一种新的 NLMO 改性策略,通过在体相中以 Zr4+ 替代 Mn4+,抑制 O2-/On- 氧化还原偶的活性,稳定 ZrO2 第二相晶格,从而有效提高 NLMO 的循环稳定性。结果表明,Zr4+ 改变了晶格中 O 的配位环境和电子结构,促使 Mn t2g 进一步分裂,从而增加了费米级附近 Mn 3d 轨道的比例。在第一次放电过程中,由于电荷平衡效应,Mn3+/Mn4+ 取代了 O2-/On- 的电化学活性,获得了高达 161 mAh g-1 的比容量。这种内外兼修的改良思路为解决 NLMO 高比能钠离子电池正极材料循环稳定性差和提高比容量提供了新的方向。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nano Energy
CHEMISTRY, PHYSICAL-NANOSCIENCE & NANOTECHNOLOGY
CiteScore
30.30
自引率
7.40%
发文量
1207
审稿时长
23 days
期刊介绍:
Nano Energy is a multidisciplinary, rapid-publication forum of original peer-reviewed contributions on the science and engineering of nanomaterials and nanodevices used in all forms of energy harvesting, conversion, storage, utilization and policy. Through its mixture of articles, reviews, communications, research news, and information on key developments, Nano Energy provides a comprehensive coverage of this exciting and dynamic field which joins nanoscience and nanotechnology with energy science. The journal is relevant to all those who are interested in nanomaterials solutions to the energy problem.
Nano Energy publishes original experimental and theoretical research on all aspects of energy-related research which utilizes nanomaterials and nanotechnology. Manuscripts of four types are considered: review articles which inform readers of the latest research and advances in energy science; rapid communications which feature exciting research breakthroughs in the field; full-length articles which report comprehensive research developments; and news and opinions which comment on topical issues or express views on the developments in related fields.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


