Metal-ligand redox in layered oxide cathodes for Li-ion batteries
IF 38.6
1区 材料科学
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
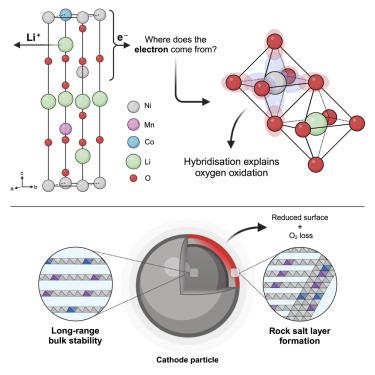
This study refutes the commonly used ionic-bonding model that demarcates transition metal (TM) and oxygen redox using an archetypal Ni-rich layered oxide cathode, LiNi0.8Mn0.1Co0.1O2. Here, charge compensation during delithiation occurs without formal (ionic) Ni oxidation. Instead, oxygen-dominated states control the redox process, facilitated by strong TM-O hybridization, forming bulk-stable 3d8L and 3d8L2 electronic states, where L is a ligand hole. Bulk O–O dimers are observed with O K-edge resonant inelastic X-ray scattering but, critically, without the long-range TM migration or void formation observed in Li-rich layered oxides. Above 4.34 V vs. Li+/Li, the cathode loses O, forming a resistive surface rock-salt layer that causes capacity fade. This highlights the importance of cathode engineering when attempting to achieve higher energy densities with layered oxide cathodes, especially in those where O dominates the charge compensation mechanism.

锂离子电池层状氧化物阴极中的金属-配体氧化还原作用
本研究利用典型的富镍层状氧化物阴极 LiNi0.8Mn0.1Co0.1O2,驳斥了划分过渡金属(TM)和氧氧化还原的常用离子键模型。在这里,脱硫过程中的电荷补偿是在没有正式(离子)镍氧化的情况下发生的。相反,氧主导态控制了氧化还原过程,并通过强 TM-O 杂化作用形成了大量稳定的 3d8L 和 3d8L2 电子态,其中 L 是配体空穴。通过 O K 边共振非弹性 X 射线散射可以观察到块状 O-O 二聚体,但重要的是,在富含锂的层状氧化物中没有观察到长程 TM 迁移或空隙形成。对 Li+/Li 的电压高于 4.34 V 时,阴极会失去 O,形成电阻性表面岩盐层,导致容量衰减。这凸显了在尝试使用层状氧化物阴极实现更高能量密度时阴极工程的重要性,尤其是在 O 主导电荷补偿机制的阴极中。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Joule
Energy-General Energy
CiteScore
53.10
自引率
2.00%
发文量
198
期刊介绍:
Joule is a sister journal to Cell that focuses on research, analysis, and ideas related to sustainable energy. It aims to address the global challenge of the need for more sustainable energy solutions. Joule is a forward-looking journal that bridges disciplines and scales of energy research. It connects researchers and analysts working on scientific, technical, economic, policy, and social challenges related to sustainable energy. The journal covers a wide range of energy research, from fundamental laboratory studies on energy conversion and storage to global-level analysis. Joule aims to highlight and amplify the implications, challenges, and opportunities of novel energy research for different groups in the field.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


