Electric syntrophy-driven modulation of Fe0-dependent microbial denitrification
IF 11.4
1区 环境科学与生态学
Q1 ENGINEERING, ENVIRONMENTAL
引用次数: 0
Abstract
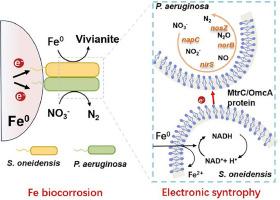
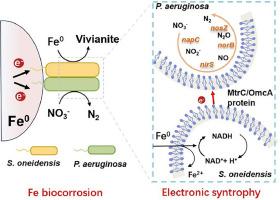
In natural or engineered anaerobic environments, iron oxidation-driven microbial denitrification plays a critical role in the water or wastewater treatment. Herein, we report a previously unidentified metallic iron (Fe0)-dependent denitrification mode driven by the electro-syntrophic interaction between electroactive microorganism and denitrifier. In a model denitrifying consortium of Shewanella oneidensis and Pseudomonas aeruginosa, we find that P. aeruginosa can accept electrons for nitrate reduction via the constructed electron transfer system of Fe0–S. oneidensis–P. aeruginosa. In the electro-syntrophic consortium, the membrane-bound CymA–OmcA–MtrC protein complexes of S. oneidensis drive the generation, transfer and consumption of electrons, thus enabling modulation of microbial metabolic activity. Specially, using Fe0 as the sole electron donor, S. oneidensis can act as a bio-engine to harvest electrons and conserve energy from Fe0 biocorrosion. Electrons released by S. oneidensis are utilized by P. aeruginosa for accomplishing microbial denitrification. Metatranscriptomics analysis demonstrated that the direct electron cross-feeding process facilitates the expression of genes encoding for denitrification enzymes, intracellular electron transfer proteins, and quorum sensing of P. aeruginosa. The Fe0-dependent electronic syntrophy in this work could provide a metabolic window for the growth of denitrifiers that is a new insight into nitrate removal or global nitrogen cycle.
电合成驱动对依赖于 Fe0 的微生物反硝化作用的调节
在自然或工程厌氧环境中,铁氧化驱动的微生物反硝化作用在水或废水处理中起着至关重要的作用。在此,我们报告了一种之前未被发现的依赖于金属铁(Fe0)的反硝化模式,这种模式是由电活性微生物和反硝化物之间的电营养相互作用驱动的。在由 Shewanella oneidensis 和铜绿假单胞菌组成的模型反硝化联合体中,我们发现铜绿假单胞菌可以通过构建的 Fe0-S. oneidensis-P. aeruginosa 电子传递系统接受电子进行硝酸盐还原。在电营养联合体中,S. oneidensis 的膜结合 CymA-OmcA-MtrC 蛋白复合物驱动电子的产生、转移和消耗,从而调节微生物的代谢活动。特别是,利用 Fe0 作为唯一的电子供体,S. oneidensis 可以充当生物发动机,从 Fe0 的生物腐蚀中收集电子并保存能量。铜绿微囊藻利用 S. oneidensis 释放的电子完成微生物脱氮。元转录组学分析表明,直接电子交叉馈送过程促进了铜绿微囊藻的反硝化酶、胞内电子传递蛋白和法定量感应等编码基因的表达。这项工作中的依赖于铁0的电子合成可能为反硝化菌的生长提供了一个新陈代谢窗口,从而为硝酸盐去除或全球氮循环提供了新的视角。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Water Research
环境科学-工程:环境
CiteScore
20.80
自引率
9.40%
发文量
1307
审稿时长
38 days
期刊介绍:
Water Research, along with its open access companion journal Water Research X, serves as a platform for publishing original research papers covering various aspects of the science and technology related to the anthropogenic water cycle, water quality, and its management worldwide. The audience targeted by the journal comprises biologists, chemical engineers, chemists, civil engineers, environmental engineers, limnologists, and microbiologists. The scope of the journal include:
•Treatment processes for water and wastewaters (municipal, agricultural, industrial, and on-site treatment), including resource recovery and residuals management;
•Urban hydrology including sewer systems, stormwater management, and green infrastructure;
•Drinking water treatment and distribution;
•Potable and non-potable water reuse;
•Sanitation, public health, and risk assessment;
•Anaerobic digestion, solid and hazardous waste management, including source characterization and the effects and control of leachates and gaseous emissions;
•Contaminants (chemical, microbial, anthropogenic particles such as nanoparticles or microplastics) and related water quality sensing, monitoring, fate, and assessment;
•Anthropogenic impacts on inland, tidal, coastal and urban waters, focusing on surface and ground waters, and point and non-point sources of pollution;
•Environmental restoration, linked to surface water, groundwater and groundwater remediation;
•Analysis of the interfaces between sediments and water, and between water and atmosphere, focusing specifically on anthropogenic impacts;
•Mathematical modelling, systems analysis, machine learning, and beneficial use of big data related to the anthropogenic water cycle;
•Socio-economic, policy, and regulations studies.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


