T6SS-associated Rhs toxin-encapsulating shells: Structural and bioinformatical insights into bacterial weaponry and self-protection
IF 4.4
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
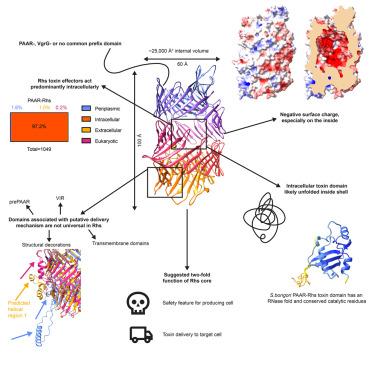
Bacteria use the type VI secretion system (T6SS) to secrete toxins into pro- and eukaryotic cells via machinery consisting of a contractile sheath and a rigid tube. Rearrangement hotspot (Rhs) proteins represent one of the most common T6SS effectors. The Rhs C-terminal toxin domain displays great functional diversity, while the Rhs core is characterized by YD repeats. We elucidate the Rhs core structures of PAAR- and VgrG-linked Rhs proteins from Salmonella bongori and Advenella mimigardefordensis, respectively. The Rhs core forms a large shell of β-sheets with a negatively charged interior and encloses a large volume. The S. bongori Rhs toxin does not lead to ordered density in the Rhs shell, suggesting the toxin is unfolded. Together with bioinformatics analysis showing that Rhs toxins predominantly act intracellularly, this suggests that the Rhs core functions two-fold, as a safety feature for the producer cell and as delivery mechanism for the toxin.
与 T6SS 相关的 Rhs 毒素包裹壳:从结构和生物信息学角度洞察细菌武器与自我保护
细菌利用 VI 型分泌系统(T6SS),通过由收缩鞘和硬管组成的机械装置向原核和真核细胞分泌毒素。重排热点(Rhs)蛋白是最常见的 T6SS 效应器之一。Rhs C 端毒素结构域显示出极大的功能多样性,而 Rhs 核心则以 YD 重复为特征。我们阐明了分别来自邦戈里沙门氏菌和米氏酵母菌的 PAAR 链接 Rhs 蛋白和 VgrG 链接 Rhs 蛋白的 Rhs 核心结构。Rhs 核心形成了一个由内部带负电荷的 β 片层组成的大外壳,并包围着一个大体积。S. bongori Rhs毒素不会导致Rhs外壳出现有序密度,这表明毒素是未折叠的。生物信息学分析表明,Rhs毒素主要在细胞内发挥作用,这表明Rhs核心具有双重功能,既是生产细胞的安全特征,也是毒素的输送机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Structure
生物-生化与分子生物学
CiteScore
8.90
自引率
1.80%
发文量
155
审稿时长
3-8 weeks
期刊介绍:
Structure aims to publish papers of exceptional interest in the field of structural biology. The journal strives to be essential reading for structural biologists, as well as biologists and biochemists that are interested in macromolecular structure and function. Structure strongly encourages the submission of manuscripts that present structural and molecular insights into biological function and mechanism. Other reports that address fundamental questions in structural biology, such as structure-based examinations of protein evolution, folding, and/or design, will also be considered. We will consider the application of any method, experimental or computational, at high or low resolution, to conduct structural investigations, as long as the method is appropriate for the biological, functional, and mechanistic question(s) being addressed. Likewise, reports describing single-molecule analysis of biological mechanisms are welcome.
In general, the editors encourage submission of experimental structural studies that are enriched by an analysis of structure-activity relationships and will not consider studies that solely report structural information unless the structure or analysis is of exceptional and broad interest. Studies reporting only homology models, de novo models, or molecular dynamics simulations are also discouraged unless the models are informed by or validated by novel experimental data; rationalization of a large body of existing experimental evidence and making testable predictions based on a model or simulation is often not considered sufficient.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


