Small gold nanoparticles for tandem cyclization/reduction and cyclization/hydroalkoxylation reactions
IF 5.9
2区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
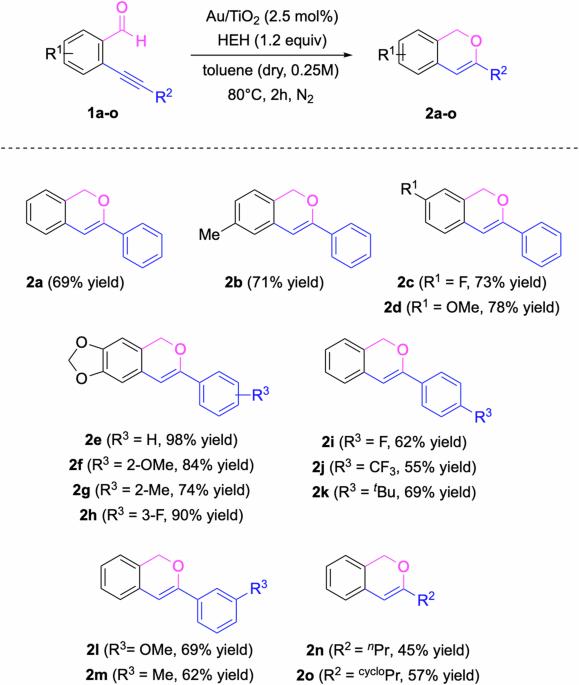
With peculiar structural features at the surface of small metal nanoparticles, some discrete sites can display catalytic behaviour similar to what could be observed with mononuclear metal catalysts in solution. We have studied the transfer of two catalytic tandem reactions from homogeneous to heterogeneous conditions. Tandem cyclisation/reduction of ortho-alkynyl benzaldehyde derivatives was successfully achieved with Au nanoparticles over TiO2 (Au NPs/TiO2) in the presence of Hantzsch ester with 45-98% yields for 15 examples (average yield: 70.4%). Similarly, tandem cyclisation/hydroalkoxylation of ortho-alkynyl benzaldehyde derivatives was successfully achieved with Au NPs/TiO2 in methanol or other alcohols with 62-96% yields for 17 examples (average yield: 84.9%). The application potential of this catalytic system was demonstrated with the total synthesis of a bioactive isochromene derivative featuring one of the developed reactions as the key step and the implementation of the tandem cyclisation/hydroalkoxylation in a continuous flow reactor, scaling up the reaction by a factor of 10 without loss of efficiency. Heterogeneous catalysis offers a range of advantages over homogeneous catalysis, but transferring a reaction from homogeneous to heterogeneous protocols is highly challenging. Here, the authors study the transfer of two catalytic tandem reactions—a cyclization/reduction and a cyclization/hydroalkoxylation—from homogeneous to heterogeneous conditions, using gold nanoparticles to generate isochromene derivatives from ortho-alkynyl benzaldehyde starting materials, and demonstrating scalability in flow.
用于串联环化/还原和环化/氢烷氧基化反应的小金纳米粒子
由于小金属纳米粒子表面的特殊结构特征,一些离散位点可以显示出与溶液中单核金属催化剂类似的催化行为。我们研究了两个催化串联反应从均相条件向异相条件的转移。在 Hantzsch 酯存在下,使用金纳米粒子在二氧化钛(Au NPs/TiO2)上成功实现了邻炔基苯甲醛衍生物的串联环化/还原反应,15 个实例的产率为 45-98%(平均产率:70.4%)。同样,利用 Au NPs/TiO2 在甲醇或其他醇中成功实现了邻炔基苯甲醛衍生物的串联环化/氢烷氧基化反应,17 个实例的产率为 62-96%(平均产率:84.9%)。该催化系统的应用潜力体现在生物活性异色烯衍生物的全合成中,其中一个关键步骤就是所开发的反应,并在连续流反应器中实现了串联环化/氢化烷氧基化反应,在不降低效率的情况下将反应放大了 10 倍。与均相催化相比,异相催化具有一系列优势,但将反应从均相协议转移到异相协议却极具挑战性。在本文中,作者研究了两个催化串联反应--环化/还原反应和环化/氢化烷氧基化反应--从均相到异相条件的转移,使用金纳米粒子从邻炔基苯甲醛起始材料生成异色烯衍生物,并展示了在流动条件下的可扩展性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Communications Chemistry
Chemistry-General Chemistry
CiteScore
7.70
自引率
1.70%
发文量
146
审稿时长
13 weeks
期刊介绍:
Communications Chemistry is an open access journal from Nature Research publishing high-quality research, reviews and commentary in all areas of the chemical sciences. Research papers published by the journal represent significant advances bringing new chemical insight to a specialized area of research. We also aim to provide a community forum for issues of importance to all chemists, regardless of sub-discipline.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


