Reaction of 6Н-5-(4-Fluorophenyl)-3-(2-pyridyl)-1,2,4-triazine with 4,5-Difluoro-1,2-dehydrobenzene
IF 0.9
4区 化学
Q4 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
Abstract
The reaction of C6-unsubstituted 5-aryl-3-(2-pyridyl)-1,2,4-triazine with generated in situ difluoroaryne intermediate (4,5-difluoro-1,2-dehydrobenzene), previously unused for this aim, was studied. New transformations of the 1,2,4-triazine nucleus were discovered, which lead, along with the domino transformation product (10-(1,2,3-triazole-3-yl)pyrido[1,2-a]indole) natural for this transformation, to the formation of unexpected products, namely 1,3,5-tris-substituted 1,6-dihydro-1,2,4-triazin-6-ol and 1H-1,2,4-triazole. The structure of the products was confirmed by physicochemical methods, including X-ray diffraction analysis.
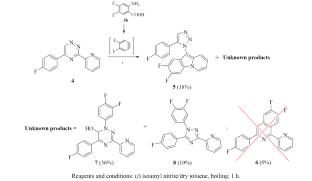
6Н-5-(4-氟苯基)-3-(2-吡啶基)-1,2,4-三嗪与 4,5-二氟-1,2-脱氢苯的反应
我们研究了 C6-未取代的 5-芳基-3-(2-吡啶基)-1,2,4-三嗪与原位生成的二氟芳基炔中间体(4,5-二氟-1,2-脱氢苯)的反应。发现了 1,2,4-三嗪核的新转化,这些转化与多米诺转化产物(10-(1,2,3-三唑-3-基)吡啶并[1,2-a]吲哚)一起形成了意想不到的产物,即 1,3,5-三取代 1,6-二氢-1,2,4-三嗪-6-醇和 1H-1,2,4-三唑。这些产物的结构已通过物理化学方法(包括 X 射线衍射分析)得到证实。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.40
自引率
22.20%
发文量
252
审稿时长
2-4 weeks
期刊介绍:
Russian Journal of General Chemistry is a journal that covers many problems that are of general interest to the whole community of chemists. The journal is the successor to Russia’s first chemical journal, Zhurnal Russkogo Khimicheskogo Obshchestva (Journal of the Russian Chemical Society ) founded in 1869 to cover all aspects of chemistry. Now the journal is focused on the interdisciplinary areas of chemistry (organometallics, organometalloids, organoinorganic complexes, mechanochemistry, nanochemistry, etc.), new achievements and long-term results in the field. The journal publishes reviews, current scientific papers, letters to the editor, and discussion papers.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


