Glucose and glutamine drive hepatitis E virus replication
Abstract
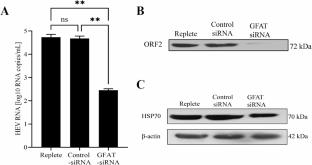
Viruses have undergone evolutionary adaptations to tune their utilization of carbon sources, enabling them to extract specific cellular substrates necessary for their replication. The lack of a reliable cell culture system and a small-animal model has hampered our understanding of the molecular mechanism of replication of hepatitis E virus (HEV) genotype 1. Our recent identification of a replicative ensemble of mutant HEV RNA libraries has allowed us to study the metabolic prerequisites for HEV replication. Initial assessments revealed increased glucose and glutamine utilization during HEV replication. Inhibition of glycolysis and glycolysis + glutaminolysis reduced the levels of HEV replication to similar levels. An integrated analysis of protein-metabolite pathways suggests that HEV replication markedly alters glycolysis, the TCA cycle, and glutamine-associated metabolic pathways. Cells supporting HEV replication showed a requirement for fructose-6-phosphate and glutamine utilization through the hexosamine biosynthetic pathway (HBP), stimulating HSP70 expression to facilitate virus replication. Observations of mannose utilization and glutamine dependence suggest a crucial role of the HBP in supporting HEV replication. Inhibition of glycolysis and HSP70 activity or knockdown of glutamine fructose-6-phosphate amidotransferase expression led to a substantial reduction in HEV RNA and ORF2 expression accompanied by a significant decrease in HSP70 levels. This study demonstrates that glucose and glutamine play critical roles in facilitating HEV replication.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


