ESIPT-induced intersystem crossing leads to tautomer fluorescence quenching for 3-mercapto-2-(4-(trifluoromethyl)phenyl)-4H-chromen-4-one molecule
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-24
DOI:10.1016/j.jphotochem.2024.116111
引用次数: 0
Abstract
The excited-state intramolecular proton-transfer (ESIPT) dynamics of 2-(4-(diethylamino)phenyl)-3-mercapto-4H-chromen-4-one (3NTF) and 3-mercapto-2-(4-(trifluoromethyl)phenyl)-4H-chromen-4-one (3FTF) have been investigated using time-dependent density functional theory (TDDFT). Upon photoexcitation, 3NTF exhibits a single fluorescence emission while 3FTF is fluorescence quenched when dissolved in cyclohexane solution. The present study reveals that both species undergo barrierless ESIPT process, and the underlying reason for fluorescence quenching in 3FTF has been elucidated. Specifically, it is concluded that intersystem crossing (ISC) is responsible for the fluorescence quenching in the 3FTF molecule due to the energy gap between the S1 and T2 states is only 0.12 eV plus large S1 → T2 spin–orbit coupling resulting in a strong interaction between the singlet and triplet states. The present study provides a reference for the fluorescence quenching associated with thiol-hydrogen bond molecules, and it is helpful for further research on ESIPT reactions of sulfur-containing molecules.
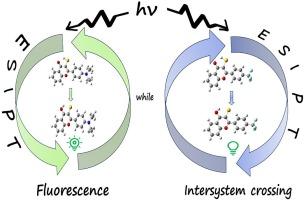
ESIPT 诱导的系统间交叉导致 3-巯基-2-(4-(三氟甲基)苯基)-4H-苯并吡喃-4-酮分子的同系物荧光淬灭
利用时间相关密度泛函理论(TDDFT)研究了 2-(4-(二乙基氨基)苯基)-3-巯基-4H-苯并吡喃-4-酮(3NTF)和 3-巯基-2-(4-(三氟甲基)苯基)-4H-苯并吡喃-4-酮(3FTF)的激发态分子内质子转移动力学。在光激发下,3NTF 发出单一荧光,而 3FTF 溶于环己烷溶液时会被淬灭荧光。本研究揭示了这两种物质都经历了无障碍 ESIPT 过程,并阐明了 3FTF 荧光淬灭的根本原因。具体来说,由于 S1 和 T2 态之间的能隙仅为 0.12 eV,加上 S1 → T2 自旋轨道耦合很大,导致单重态和三重态之间的相互作用很强,因此得出结论认为系统间交叉(ISC)是 3FTF 分子中荧光淬灭的原因。本研究为硫醇-氢键分子相关的荧光淬灭提供了参考,有助于进一步研究含硫分子的 ESIPT 反应。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


