Pyrimidine derivative mimicking the locked enol form of avobenzone acts as a photostable UVAII and UVB filter
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-19
DOI:10.1016/j.jphotochem.2024.116099
引用次数: 0
Abstract
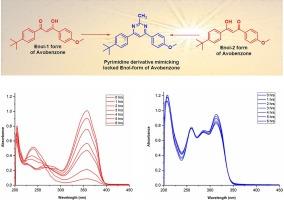
The broad UVA (UVAI and UVAII) filtering activity of the sunscreen agent avobenzone is due to its enolic forms which undergoes ketonization followed by degradation upon exposure to sunlight/radiation. The current report aims to lock the enolic forms of avobenzone through chemical derivatization that preserves the chelated intramolecular hydrogen bond geometry. The pyrimidine derivative mimicking both the enol-1 and enol-2 forms of avobenzone has been synthesized and evaluated for it photostability under natural sunlight by UV spectroscopy. The avobenzone pyrimidine derivative acts as a broad-spectrum UVAII, and UVB filter and exhibits unprecedented photostability under sunlight. The new derivative of avobenzone is a valuable additive to the tool kit of chemical UV filters and its poor skin permeability relative to the native avobenzone may be an advantage for cosmetics.
模仿阿伏苯宗锁烯醇形式的嘧啶衍生物可用作光稳定性 UVAII 和 UVB 过滤器
防晒剂阿伏苯宗之所以具有广泛的 UVA(UVAI 和 UVAII)过滤活性,是因为它的烯醇形式在暴露于阳光/辐射时会发生酮化,然后发生降解。本报告旨在通过化学衍生化来锁定阿伏苯宗的烯醇形式,从而保留螯合分子内氢键的几何形状。我们合成了模仿阿伏苯宗烯醇-1 和烯醇-2 形式的嘧啶衍生物,并通过紫外光谱评估了其在自然光下的光稳定性。阿伏苯宗嘧啶衍生物是一种广谱 UVAII 和 UVB 过滤器,在阳光下表现出前所未有的光稳定性。这种新的阿伏苯宗衍生物是化学紫外线过滤器工具包中的一种有价值的添加剂,与原生阿伏苯宗相比,它的皮肤渗透性较差,这可能是其在化妆品方面的一个优势。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


