Remarkable NH3 gas sensing performance of spray deposited Tb doped WO3 thin films at room temperature
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-17
DOI:10.1016/j.jphotochem.2024.116087
引用次数: 0
Abstract
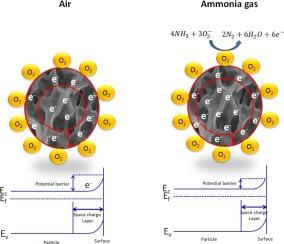
To evade potential health hazards associated with exposure to NH3, there has been an increasing demand for efficient gas sensors operating at room temperature (RT). In this study, thin films of Tb-doped (0–5 wt%) WO3 synthesized by spray pyrolysis technique are used as a novel material for sensing NH3 gas. The X-ray diffraction (XRD) pattern identified the hexagonal crystal system of WO3 films and increased crystallinity for the 2 wt% Tb-doing concentration. Field emission scanning electron microscopy (FE-SEM) unveiled the distinct surface morphology of mesh-like porous structures for Tb-doped films suitable for the target gas adsorption/desorption process. Multiple photoluminescence (PL) emission peaks indicate the presence of defect states, including defect energy levels created by oxygen vacancies (Ov). Optical analysis indicated shrinkage of the bandgap of WO3 thin films for doping levels up to 2 wt%. Among all gas sensors, 2 wt% Tb-doped WO3 exhibited exceptionally high response and low response time at 250 ppm NH3 concentrations measured at room temperature (RT). The sensor’s performance for NH3 gas sensing is compared with previous reports on WO3-based NH3 sensors. The gas sensing mechanism in WO3 is also briefly discussed.
室温下喷雾沉积掺杂 Tb 的 WO3 薄膜的显著 NH3 气体传感性能
为了避免接触 NH3 对健康造成的潜在危害,人们对可在室温 (RT) 下工作的高效气体传感器的需求与日俱增。本研究采用喷雾热解技术合成了掺杂 Tb(0-5 wt%)的 WO3 薄膜,并将其作为一种新型材料用于感应 NH3 气体。X 射线衍射(XRD)图谱确定了 WO3 薄膜的六方晶系,并且掺杂 2 wt% Tb 浓度时结晶度增加。场发射扫描电子显微镜(FE-SEM)揭示了掺镱薄膜的独特表面形态,即适合目标气体吸附/解吸过程的网状多孔结构。多个光致发光(PL)发射峰表明存在缺陷态,包括氧空位(Ov)产生的缺陷能级。光学分析表明,当掺杂水平达到 2 wt% 时,WO3 薄膜的带隙会缩小。在所有气体传感器中,掺杂 2 wt% Tb 的 WO3 在室温(RT)下测量浓度为 250 ppm 的 NH3 时表现出极高的响应速度和极短的响应时间。该传感器的 NH3 气体传感性能与之前有关基于 WO3 的 NH3 传感器的报道进行了比较。此外,还简要讨论了 WO3 的气体传感机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


