Kinetics of CO2 hydrate formation in clayey sand sediments: Implications for CO2 sequestration
0 ENERGY & FUELS
引用次数: 0
Abstract
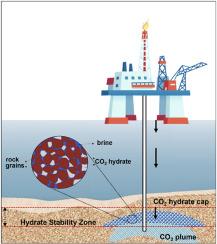
Hydrate-based CO2 sequestration beneath oceanic sediments is an emerging technique that involves the injection of CO2 into the hydrate stability zone (HSZ) beneath the seabed, forming hydrate cap that structurally traps the injected CO2 and reduces the risk of leaking CO2 from the storage sediment. Gas hydrates are adequately stable in sand sediments saturated with fresh water; however, the salinity of oceanic water impairs hydrate formation kinetics, stability, and CO2 storage capacity. In addition to sandstones, marine sediments are composed of many clay minerals that could affect hydrate formation. Therefore, this study experimentally simulates CO2 injection into sand and clayey sand sediments to assess the potential of CO2 hydrate formation. CO2 hydrates are formed inside a high-pressure reactor, which contains unconsolidated sediment bed/pack (silica sand; mixed sand with bentonite clay: 5 wt% and 10 wt%), saturated with de-ionized water or brine (3.3 wt% NaCl). Hydrate formation experiments were performed at 4 MPa pressure and 274.15 K temperature. Results show that CO2 hydrate formed within the sand sediment, with induction times of 6 and 8.5 h, for the de-ionized and brine systems, respectively. CO2 gas mole uptake in the de-ionized system was 71.54 mmol/mol however, in the brine system the gas uptake was 56.95 mmol/mol. Hence this 20.4% reduction in the gas uptake indicated the inhibition effect of salinity. In contrast, in the brine-saturated 5 wt% clay-sand sediment, the induction time was 6.5 h, indicating the promoting effect of the nano-sized clay particles. However, the gas uptake in this brine-saturated clay-sand sediment was reduced by 45.51% compared to the brine-saturated sand sediment. Increasing the clay content to 10 wt% prevented CO2 hydrate formation due to porosity reduction. Moreover, de-ionized water in clayey sand sediments prevented hydrate formation due to clay swelling. Finally, CO2 hydrate formation at the end of each experiment was visually confirmed.
粘性砂沉积物中二氧化碳水合物的形成动力学:二氧化碳封存的意义
基于水合物的海洋沉积物下二氧化碳封存技术是一种新兴技术,它将二氧化碳注入海床下的水合物稳定区(HSZ),形成水合物盖,从结构上封存注入的二氧化碳,降低二氧化碳从封存沉积物中泄漏的风险。在淡水饱和的砂质沉积物中,天然气水合物具有足够的稳定性;但海水的盐度会影响水合物的形成动力学、稳定性和二氧化碳封存能力。除砂岩外,海洋沉积物还由许多可能影响水合物形成的粘土矿物组成。因此,本研究通过实验模拟将二氧化碳注入砂岩和含粘土的砂岩沉积物,以评估二氧化碳水合物形成的潜力。二氧化碳水合物是在高压反应器内形成的,该反应器包含未固结沉积物床/包(硅砂;含膨润土的混合砂:5 wt% 和 10 wt%),用去离子水或盐水(3.3 wt% 氯化钠)饱和。水合物形成实验在 4 兆帕压力和 274.15 K 温度下进行。结果表明,二氧化碳水合物在砂沉积物中形成,去离子水和盐水系统的诱导时间分别为 6 和 8.5 小时。在去离子系统中,二氧化碳气体摩尔吸收量为 71.54 mmol/mol,而在盐水系统中,气体吸收量为 56.95 mmol/mol。因此,气体吸收量减少的 20.4% 表明盐度具有抑制作用。相反,在盐水饱和的 5 wt% 粘土-砂沉积物中,诱导时间为 6.5 小时,表明纳米级粘土颗粒具有促进作用。然而,与盐水饱和的砂沉积物相比,盐水饱和的粘土砂沉积物的气体吸收率降低了 45.51%。将粘土含量提高到 10 wt%,可防止因孔隙率降低而形成二氧化碳水合物。此外,粘土质砂质沉积物中的去离子水可防止粘土膨胀导致的水合物形成。最后,在每个实验结束时,二氧化碳水合物的形成都得到了目测确认。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


