Hydrogen purification via hydrate-based methods: Insights into H2-CO2-CO hydrate structures, thermodynamics, and kinetics
0 ENERGY & FUELS
引用次数: 0
Abstract
Hydrate-based H2 purification is promising for removing impurities owing to the cage-like structures formed by water molecules. Understanding the competition mechanism of multicomponent gas molecule in hydrate is critical for improving gas purification efficiency. This study investigated the hydrate structure, thermodynamics, and kinetics of ternary gas mixture (H2–CO2–CO) from natural gas reforming products. The results identified H2–CO2–CO hydrate as type sI and determined the lattice parameters using X-ray diffraction. Raman spectroscopy results confirmed the presence of H2, CO2, and CO gas molecules in the hydrate phase. Increasing pressure, compared to cooling, enhanced CO2 content in hydrate phase. The phase equilibrium conditions and average hydrate dissociation enthalpy (53.47 J/g) were determined using a high-pressure microcalorimeter. Gas chromatography identified that the content of CO2 in the hydrate was the highest, followed by H2, and CO was the least abundant. The molecule dynamics simulation results show CO2 molecule numbers reflected trends in 51262 cages, H2 numbers correlated with changes in 512 cages, while CO numbers showed insignificant variation. Under the gas composition studied, the ability of gas molecules to be incorporated within hydrates decreases in the following order: CO2, H2, and CO.
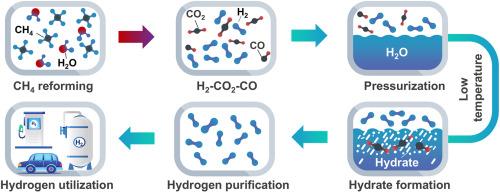
通过基于水合物的方法提纯氢气:对 H2-CO2-CO 水合物结构、热力学和动力学的见解
由于水分子形成的笼状结构,基于水合物的 H2 净化技术有望去除杂质。了解多组分气体分子在水合物中的竞争机制对于提高气体净化效率至关重要。本研究对天然气重整产物三元气体混合物(H2-CO2-CO)的水合物结构、热力学和动力学进行了研究。结果确定 H2-CO2-CO 水合物为 sI 型,并利用 X 射线衍射确定了其晶格参数。拉曼光谱结果证实水合物相中存在 H2、CO2 和 CO 气体分子。与冷却相比,增加压力会提高水合物相中的 CO2 含量。使用高压微量热仪测定了相平衡条件和平均水合物解离焓(53.47 J/g)。气相色谱法确定水合物中 CO2 的含量最高,其次是 H2,CO 的含量最低。分子动力学模拟结果表明,二氧化碳分子数量反映了 51262 个笼子的变化趋势,H2 数量与 512 个笼子的变化相关,而 CO 数量变化不大。在所研究的气体成分中,气体分子融入水合物的能力按以下顺序降低:CO2、H2 和 CO。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


