Adsorption of selected oxyanions from aqueous solution using Benzethonium chloride modified zeolite 4A: Artificial neural network approach
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
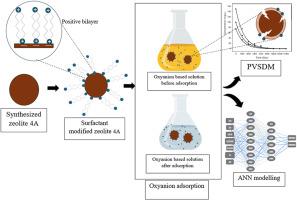
Over the years, a wide range of cationic surfactants have been used for the modification of zeolite surface chemistry. In this research study, the synthesized surfactant modified zeolites were characterized using scanning electron microscopy-energy dispersive spectrometry (SEM-EDS), Fourier-transformed infrared spectroscopy (FTIR), Brunauer-Emmet-Teller (BET) and zeta potential analysis. Cr (VI) and Mo (VI). After modification, an increase in Cr (VI) and Mo (VI) oxyanion removal efficiency of zeolite 4A from 22.99 % to 96.3 % and 51.85 %–85.61 % has been observed. The pore volume diffusion and surface diffusion coefficients Dp and DS were in order of 10−12 cm2 s−1. The mass transfer coefficient Kf were in the order of 10−5 cm s−1. Cr (VI) and Mo (VI) adsorption on BZT-zeolite is good agreement with Langmuir and Sips models. The Cr (VI) and Mo (VI) uptake capacities are approximately equivalent to 3 mg/g. The highest enthalpy changes (ΔH°) for the removal of Cr (VI) and Mo (VI) were −105.429 and −53.243 kJ/mol respectively. The entropy changes (ΔS°) were −0.335 and −0.163 kJ/mol for the removal of Cr (VI) and Mo (VI) respectively. The free energies of adsorption of Cr (VI) and Mo (VI) were lower than zero. Hence, Cr (VI) and Mo (VI) adsorption process was found to be spontaneous and exothermic. ANN shows excellent modelling for the adsorption process with relatively error values lower than 10 %.
使用氯化苄改性沸石 4A 吸附水溶液中的某些氧阴离子:人工神经网络方法
多年来,各种阳离子表面活性剂被广泛用于沸石表面化学改性。在这项研究中,使用扫描电子显微镜-能量色散光谱法(SEM-EDS)、傅立叶变换红外光谱法(FTIR)、Brunauer-Emmet-Teller(BET)和 zeta 电位分析法对合成的表面活性剂改性沸石进行了表征。铬(VI)和钼(VI)。经过改性后,沸石 4A 对 Cr (VI) 和 Mo (VI) 氧阴离子的去除率分别从 22.99 % 和 51.85 % 提高到 96.3 % 和 85.61 %。孔隙体积扩散系数 Dp 和表面扩散系数 DS 为 10-12 cm2 s-1。传质系数 Kf 的数量级为 10-5 cm s-1。BZT-zeolite 对 Cr (VI) 和 Mo (VI) 的吸附与 Langmuir 和 Sips 模型十分吻合。铬(VI)和钼(VI)的吸附容量大约相当于 3 毫克/克。六价铬和六价钼的最高焓变(ΔH°)分别为-105.429 和-53.243 kJ/mol。去除铬 (VI) 和钼 (VI) 的熵变 (ΔS°) 分别为 -0.335 和 -0.163 kJ/mol。铬(六价铬)和钼(六价钼)的吸附自由能低于零。因此,铬(VI)和钼(VI)的吸附过程是自发和放热的。ANN 对吸附过程的建模效果极佳,误差值相对低于 10%。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


