Injectable self-healing alginate/PEG hydrogels cross-linked via thiol-Michael addition bonds for hemostasis and wound healing
IF 12.5
1区 化学
Q1 CHEMISTRY, APPLIED
引用次数: 0
Abstract
In this study, an alginate/PEG hydrogel was developed via a thiol-Michael addition reaction between oxidized quinone of catechols on dopamine-grafted sodium alginate (SA-DA) and sulfhydryl groups of 4-arm polyethylene glycol tetra-thiol (4-arm PEG-SH) under mildly basic conditions. Through the formation of thiol-terminated catechol groups, the accompanying oxidized catechols are reduced, significantly strengthening the internal network structure of the hydrogel and improving tissue adhesion. Meanwhile, the hydrogels have excellent self-healing properties due to the dynamic non-covalent bonds between the groups. Adjustment of hydrogel properties by varying the mass ratio of two hydrogel precursors. Due to the high content of thiol-terminated catechol groups, the Gel 3 exhibited good tissue adhesion, rapid self-healing ability, and other multifunctions beneficial to wound healing, including killing of E. coli and S. aureus, rapid hemostasis and promoting migration of L929 cells. The full-thickness skin wound model shows that the hydrogel dressing significantly accelerated wound contraction, with increased granulation tissue thickness, collagen disposition, and enhanced vascularization, thus promoting wound healing. Therefore, the thiol-Michael addition reaction is an effective method for creating multifunctional hydrogels, and the injectable self-healing alginate/PEG hydrogels prepared in this way could be used in the biomedical area as wound healing dressing materials.
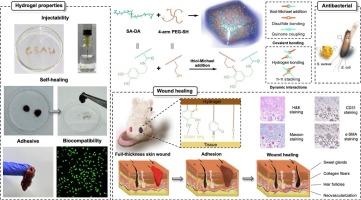
通过硫醇-迈克尔加成键交联的可注射自愈合藻酸盐/聚乙二醇水凝胶用于止血和伤口愈合
本研究在弱碱性条件下,通过多巴胺接枝海藻酸钠(SA-DA)上儿茶酚的氧化醌与 4-臂聚乙二醇四硫醇(4-臂 PEG-SH)的巯基之间的硫醇-迈克尔加成反应,开发了一种海藻酸盐/PEG 水凝胶。通过形成硫醇端邻苯二酚基团,伴随的氧化邻苯二酚被还原,从而大大加强了水凝胶的内部网络结构,提高了组织粘附性。同时,由于基团间存在动态非共价键,水凝胶具有优异的自愈性能。通过改变两种水凝胶前体的质量比来调整水凝胶的特性。由于硫醇端邻苯二酚基团的含量较高,凝胶 3 具有良好的组织粘附性、快速自愈合能力和其他有利于伤口愈合的多功能性,包括杀死大肠杆菌和金黄色葡萄球菌、快速止血和促进 L929 细胞迁移。全厚皮肤伤口模型显示,水凝胶敷料能明显加速伤口收缩,肉芽组织厚度增加,胶原配置增加,血管生成增强,从而促进伤口愈合。因此,硫醇-迈克尔加成反应是制备多功能水凝胶的有效方法,以此制备的可注射自愈合海藻酸/PEG 水凝胶可作为伤口愈合敷料材料应用于生物医学领域。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Carbohydrate Polymers
化学-高分子科学
CiteScore
22.40
自引率
8.00%
发文量
1286
审稿时长
47 days
期刊介绍:
Carbohydrate Polymers stands as a prominent journal in the glycoscience field, dedicated to exploring and harnessing the potential of polysaccharides with applications spanning bioenergy, bioplastics, biomaterials, biorefining, chemistry, drug delivery, food, health, nanotechnology, packaging, paper, pharmaceuticals, medicine, oil recovery, textiles, tissue engineering, wood, and various aspects of glycoscience.
The journal emphasizes the central role of well-characterized carbohydrate polymers, highlighting their significance as the primary focus rather than a peripheral topic. Each paper must prominently feature at least one named carbohydrate polymer, evident in both citation and title, with a commitment to innovative research that advances scientific knowledge.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


